Intratesticular creatine maintains spermatogenesis by defining tight junctions
- PMID: 39730393
- PMCID: PMC11680987
- DOI: 10.1038/s41598-024-77986-3
Intratesticular creatine maintains spermatogenesis by defining tight junctions
Abstract
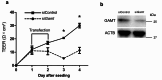
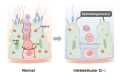
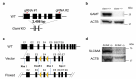
One in five couples who wish to conceive is infertile, and half of these couples have male infertility. However, the causes of male infertility are still largely unknown. Creatine is stored in the body as an energy buffer, and the testes are its second-largest reservoir after muscles. Further, even though intratesticular creatine levels have long been known to decrease in male patients with infertility, its role in the testis is unknown. We investigated the intratesticular role of creatine, specifically in the context of the creatine synthesizing enzyme Gamt, and the creatine transporter Slc6a8. The Slc6a8 knockout mice showed no abnormalities in spermatogenesis. While Gamt knockout mice formed spermatozoa, they demonstrated reduced sperm count and decreased sperm motility and fertilization rate. Additionally, intratesticular creatine in Gamt knockout mice was significantly decreased, resulting in the disruption of tight junctions, which could be rectified by creatine supplementation, as was evidenced by the improved sperm count and fertilization rate in these mice. In conclusion, we identified creatine as being required for the maintenance of the tight junction in the testis.
Keywords: Gamt; Creatine; Male infertility; Testis-blood-barrier.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Molecular analysis of guanidinoacetate-n-methyltransferase (GAMT) and creatine transporter (SLC6A8) gene by using denaturing high pressure liquid chromatography (DHPLC) as a possible source of human male infertility.Pak J Pharm Sci. 2011 Jan;24(1):75-9. Pak J Pharm Sci. 2011. PMID: 21190923
-
Creatine synthesis and exchanges between brain cells: What can be learned from human creatine deficiencies and various experimental models?Amino Acids. 2016 Aug;48(8):1877-95. doi: 10.1007/s00726-016-2189-0. Epub 2016 Feb 10. Amino Acids. 2016. PMID: 26861125 Review.
-
Creatine biosynthesis and transport in health and disease.Biochimie. 2015 Dec;119:146-65. doi: 10.1016/j.biochi.2015.10.022. Epub 2015 Nov 2. Biochimie. 2015. PMID: 26542286 Review.
-
ClinGen variant curation expert panel recommendations for classification of variants in GAMT, GATM and SLC6A8 for cerebral creatine deficiency syndromes.Mol Genet Metab. 2024 May;142(1):108362. doi: 10.1016/j.ymgme.2024.108362. Epub 2024 Mar 2. Mol Genet Metab. 2024. PMID: 38452609 Free PMC article.
-
Gene delivery of AGAT and GAMT boosts creatine levels in creatine transporter deficiency patient fibroblasts.PLoS One. 2025 May 8;20(5):e0319350. doi: 10.1371/journal.pone.0319350. eCollection 2025. PLoS One. 2025. PMID: 40338959 Free PMC article.
Cited by
-
The synergic impact of decellularized testis scaffold and extracellular vesicles derived from human semen on spermatogonial stem cell survival and differentiation.Biomed Eng Online. 2025 Jul 26;24(1):94. doi: 10.1186/s12938-025-01424-2. Biomed Eng Online. 2025. PMID: 40713602 Free PMC article.
References
-
- Minhas, S. et al. European Association of Urology Guidelines on male sexual and Reproductive Health: 2021 update on male infertility. Eur. Urol.80, 603–620 (2021). - PubMed
-
- Piechka, A., Sparanese, S., Witherspoon, L., Hach, F. & Flannigan, R. Molecular mechanisms of cellular dysfunction in testes from men with non-obstructive azoospermia. Nat. Rev. Urol.10.1038/s41585-023-00837-9 (2023). - PubMed
-
- Kazak, L. & Cohen, P. Creatine metabolism: energy homeostasis, immunity and cancer biology. Nat. Rev. Endocrinol.16, 421–436 (2020). - PubMed
-
- Wyss, M. & Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev.80, 1107–1213 (2000). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

