Male and female behavioral variability and morphine response in C57BL/6J, DBA/2J, and their BXD progeny following chronic stress exposure
- PMID: 39730457
- PMCID: PMC11680947
- DOI: 10.1038/s41598-024-80767-7
Male and female behavioral variability and morphine response in C57BL/6J, DBA/2J, and their BXD progeny following chronic stress exposure
Abstract
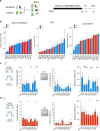
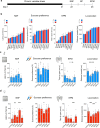
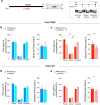
Drug addiction is a multifactorial syndrome in which genetic predispositions and exposure to environmental stressors constitute major risk factors for the early onset, escalation, and relapse of addictive behaviors. While it is well known that stress plays a key role in drug addiction, the genetic factors that make certain individuals particularly sensitive to stress and, thereby, more vulnerable to becoming addicted are unknown. In an effort to test a complex set of gene x environment interactions-specifically gene x chronic stress-here we leveraged a systems genetics resource: BXD recombinant inbred mice (BXD5, BXD8, BXD14, BXD22, BXD29, and BXD32) and their parental mouse lines, C57BL/6J and DBA/2J. Utilizing the chronic social defeat stress (CSDS) and chronic variable stress (CVS) paradigms, we first showed sexual dimorphism in social and exploratory behaviors between the mouse strains. Further, we observed an interaction between genetic background and vulnerability to prolonged exposure to non-social stressors. Finally, we found that DBA/2J and C57BL/6J mice pre-exposed to stress displayed differences in morphine sensitivity. Our results support the hypothesis that genetic variation influences chronic stress-induced behavioral outcomes such as social and approach-avoidance behaviors, reward responses, as well as morphine sensitivity, and is likely to modulate the development of drug addiction.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

Update of
-
Male and female variability in response to chronic stress and morphine in C57BL/6J, DBA/2J, and their BXD progeny.bioRxiv [Preprint]. 2024 Feb 28:2024.02.23.581795. doi: 10.1101/2024.02.23.581795. bioRxiv. 2024. Update in: Sci Rep. 2024 Dec 28;14(1):30785. doi: 10.1038/s41598-024-80767-7. PMID: 38464110 Free PMC article. Updated. Preprint.
Similar articles
-
Male and female variability in response to chronic stress and morphine in C57BL/6J, DBA/2J, and their BXD progeny.bioRxiv [Preprint]. 2024 Feb 28:2024.02.23.581795. doi: 10.1101/2024.02.23.581795. bioRxiv. 2024. Update in: Sci Rep. 2024 Dec 28;14(1):30785. doi: 10.1038/s41598-024-80767-7. PMID: 38464110 Free PMC article. Updated. Preprint.
-
Environmental enrichment influences novelty reactivity, novelty preference, and anxiety via distinct genetic mechanisms in C57BL/6J and DBA/2J mice.Sci Rep. 2021 Feb 16;11(1):3928. doi: 10.1038/s41598-021-83574-6. Sci Rep. 2021. PMID: 33594184 Free PMC article.
-
Genetic differences in the rewarding and activating effects of morphine and ethanol.Psychopharmacology (Berl). 1992;107(2-3):385-93. doi: 10.1007/BF02245166. Psychopharmacology (Berl). 1992. PMID: 1352057
-
Forebrain PENK and PDYN gene expression levels in three inbred strains of mice and their relationship to genotype-dependent morphine reward sensitivity.Psychopharmacology (Berl). 2010 Feb;208(2):291-300. doi: 10.1007/s00213-009-1730-1. Epub 2009 Dec 9. Psychopharmacology (Berl). 2010. PMID: 19997907
-
Adolescent intermittent alcohol exposure produces strain-specific cross-sensitization to nicotine and other behavioral adaptations in adulthood in C57BL/6J and DBA/2J mice.Pharmacol Biochem Behav. 2023 Nov;232:173655. doi: 10.1016/j.pbb.2023.173655. Epub 2023 Oct 5. Pharmacol Biochem Behav. 2023. PMID: 37802393 Free PMC article.
Cited by
-
Cross-species dissection of the modular role of the ventral tegmental area in depressive disorders.Neuroscience. 2025 Mar 17;569:248-266. doi: 10.1016/j.neuroscience.2025.02.008. Epub 2025 Feb 4. Neuroscience. 2025. PMID: 39914519 Review.
References
-
- Shurtleff, D., Sasek, C. & Kautz, M. Sponsor’s foreword: NIDA at forty. Neuropharmacology76(Pt B), 195–197 (2014). - PubMed
-
- Kendler, K. S., Karkowski, L. M., Neale, M. C. & Prescott, C. A. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Arch. Gen. Psychiatry57, 261–269 (2000). - PubMed
-
- Tsuang, M. T., Bar, J. L., Harley, R. M. & Lyons, M. J. The harvard twin study of substance abuse: what we have learned. Harv. Rev. Psychiatry9, 267–279 (2001). - PubMed
-
- Carlezon, W. A. J., Duman, R. S. & Nestler, E. J. The many faces of CREB. Trends Neurosci.28, 436–445 (2005). - PubMed
MeSH terms
Substances
Grants and funding
- 24AARG-NTF-1198746/ALZ/Alzheimer's Association/United States
- R01 MH120514/MH/NIMH NIH HHS/United States
- R01 MH133299/MH/NIMH NIH HHS/United States
- B2303012/Shenzhen Medical Research Fund
- T2250710685/Research Fund for International Senior Scientists
- 2021ZD0202902/National Key Research and Development Program of China
- R01 MH127820/MH/NIMH NIH HHS/United States
- J20220127/Shenzhen Natural Science Foundation
- P01 DA047233/DA/NIDA NIH HHS/United States
- R01 MH104559/MH/NIMH NIH HHS/United States
- KQTD20221101093608028/Science and Technology Research and Development Foundation of Shenzhen
- R01MH051399/MH/NIMH NIH HHS/United States
- P01DA047233/DA/NIDA NIH HHS/United States
- 29699 (CM)/NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation
- T32 MH135853/MH/NIMH NIH HHS/United States
- R01 MH051399/MH/NIMH NIH HHS/United States
- R01MH120514/MH/NIMH NIH HHS/United States
- 31194 (LFP)/NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation
- ZDSYS20220606100606014/Shenzhen Key Laboratory of Precision Diagnosis and Treatment of Depression
- 2021ZD0202900/National Key Research and Development Program of China
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

