Longitudinal analysis of SARS-CoV-2 IgG antibody durability in Puerto Rico
- PMID: 39730470
- PMCID: PMC11681247
- DOI: 10.1038/s41598-024-80465-4
Longitudinal analysis of SARS-CoV-2 IgG antibody durability in Puerto Rico
Abstract
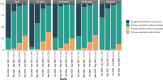
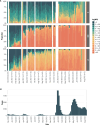
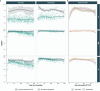
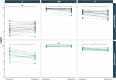
Understanding the dynamics of antibody responses following vaccination and SARS-CoV-2 infection is important for informing effective vaccination strategies and other public health interventions. This study investigates SARS-CoV-2 antibody dynamics in a Puerto Rican cohort, analyzing how IgG levels vary by vaccination status and previous infection. We assess waning immunity and the distribution of hybrid immunity with the aim to inform public health strategies and vaccination programs in Puerto Rico and similar settings. We conducted a prospective, longitudinal cohort study to identify SARS-CoV-2 infections and related outcomes in Ponce, Puerto Rico, from June 2020-August 2022. Participants provided self-collected nasal swabs every week and serum every six months for RT-PCR and IgG testing, respectively. IgG reactivity against nucleocapsid (N) antigens, which generally indicate previous infection, and spike (S1) and receptor-binding domain (RBD) antigens, which indicate history of either infection or vaccination, was assessed using the Luminex Corporation xMAP® SARS-CoV-2 Multi-Antigen IgG Assay. Prior infection was defined by positive RT-PCRs, categorized by the predominant circulating SARS-CoV-2 variant at the event time. Demographic information, medical history, and COVID-19 vaccination history were collected through standardized questionnaires. Of 882 participants included in our analysis, 34.0% experienced at least one SARS-CoV-2 infection, with most (78.7%) occurring during the Omicron wave (December 2021 onwards). SARS-CoV-2 antibody prevalence increased over time, reaching 98.4% by the final serum collection, 67.0% attributable to vaccination alone, 1.6% from infection alone, and 31.4% from both. Regardless of prior infection status, RBD and S1 IgG levels gradually declined following two vaccine doses. A third dose boosted these antibody levels and showed a slower decline over time. N-antibody levels peaked during the Omicron surge and waned over time. Vaccination in individuals with prior SARS-CoV-2 infection elicited the highest and most durable antibody responses. N or S1 seropositivity was associated with lower odds of a subsequent positive PCR test during the Omicron period, with N antibodies showing a stronger association. By elucidating the differential decay of RBD and S1 antibodies following vaccination and the complexities of N-antibody response following infection, this study in a Puerto Rican cohort strengthens the foundation for developing targeted interventions and public health strategies.
Keywords: Antibody dynamics; COVID-19; Caribbean; Humoral immunity; Omicron; Vaccination.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Figures
Similar articles
-
Diversified humoral immunity and impacts of booster vaccines: SARS-CoV-2 antibody profile and Omicron BA.2 neutralization before and after first or second boosters.Microbiol Spectr. 2024 Oct 3;12(10):e0060524. doi: 10.1128/spectrum.00605-24. Epub 2024 Aug 20. Microbiol Spectr. 2024. PMID: 39162540 Free PMC article.
-
Individuals Infected with SARS-CoV-2 Prior to COVID-19 Vaccination Maintain Vaccine-Induced RBD-Specific Antibody Levels and Viral Neutralization Activity for One Year.Viruses. 2025 Apr 29;17(5):640. doi: 10.3390/v17050640. Viruses. 2025. PMID: 40431652 Free PMC article.
-
Persistence and decay of neutralizing antibody responses elicited by SARS-CoV-2 infection and hybrid immunity in a Canadian cohort.Microbiol Spectr. 2025 Apr;13(4):e0133324. doi: 10.1128/spectrum.01333-24. Epub 2025 Feb 19. Microbiol Spectr. 2025. PMID: 39969224 Free PMC article.
-
A Meta-analysis of Severe Acute Respiratory Syndrome Coronavirus 2 Anti-spike Immunoglobulin G Antibody Durability up to 9 Months After Full Vaccination in Adults.Clin Lab Med. 2025 Mar;45(1):111-136. doi: 10.1016/j.cll.2024.10.007. Epub 2024 Dec 20. Clin Lab Med. 2025. PMID: 39892931 Review.
-
SARS-CoV-2 Vaccine-Induced Humoral Immunity in Immunocompetent European Adults: A Systematic Review.Microorganisms. 2025 Feb 27;13(3):535. doi: 10.3390/microorganisms13030535. Microorganisms. 2025. PMID: 40142428 Free PMC article. Review.
Cited by
-
Factors modulating maternofetal transfer of IgG antibodies following SARS-CoV-2 gestational infection.Rev Inst Med Trop Sao Paulo. 2025 Apr 14;67:e29. doi: 10.1590/S1678-9946202567029. eCollection 2025. Rev Inst Med Trop Sao Paulo. 2025. PMID: 40243801 Free PMC article.
References
-
- Dehgani-Mobaraki, P. et al. Long-term persistence of IgG antibodies in recovered COVID-19 individuals at 18 months post-infection and the impact of two-dose BNT162b2 (Pfizer-BioNTech) mRNA vaccination on the antibody response: Analysis using fixed-effects linear regression model. Virology578, 111–116. 10.1016/j.virol.2022.12.003 (2023). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

