Quinoline-thiosemicarbazone-1,2,3-triazole-acetamide derivatives as new potent α-glucosidase inhibitors
- PMID: 39730503
- PMCID: PMC11680592
- DOI: 10.1038/s41598-024-81668-5
Quinoline-thiosemicarbazone-1,2,3-triazole-acetamide derivatives as new potent α-glucosidase inhibitors
Abstract
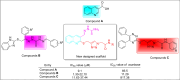
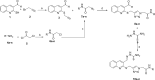
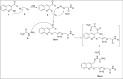
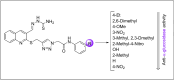
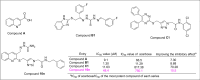
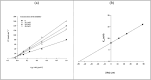
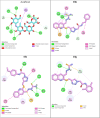
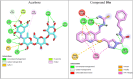
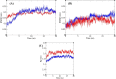
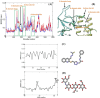
In this work, a novel series of quinoline-thiosemicarbazone-1,2,3-triazole-aceamide derivatives 10a-n as new potent α-glucosidase inhibitors was designed, synthesized, and evaluated. All the synthesized derivatives 10a-n were more potent than acarbose (positive control). Representatively, (E)-2-(4-(((3-((2-Carbamothioylhydrazineylidene)methyl)quinolin-2-yl)thio)methyl)-1H-1,2,3-triazol-1-yl)-N-phenethylacetamide (10n), as the most potent entry, with IC50 = 48.4 µM was 15.5-times more potent than acarbose. According to kinetic study, compound 10n was a competitive inhibitor against α-glucosidase. This compound formed the desired interactions with important residues of the binding pocket of α-glucosidase with favorable binding energy in the molecular docking and molecular dynamics. Compounds 10n, 10e, and 10 g as the most potent compounds among the synthesized compounds were evaluated in term of pharmacokinetics and toxicity via online servers. These evaluations predicted that compounds 10n, 10e, and 10 g had good pharmacokinetic properties and toxicity profile.
Keywords: 1,2,3-Triazole; Quinoline; Thiosemicarbazone; α-Glucosidase inhibitors.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable.
Figures
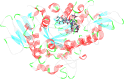
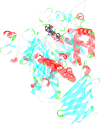
References
-
- Roglic, G. WHO Global report on diabetes: A summary. Int. J. Noncommun. Dis.1, 3–8 (2016). - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

