Development and characterization of fluorescent cholesteryl probes with enhanced solvatochromic and pH-sensitive properties for live-cell imaging
- PMID: 39730504
- PMCID: PMC11680847
- DOI: 10.1038/s41598-024-80958-2
Development and characterization of fluorescent cholesteryl probes with enhanced solvatochromic and pH-sensitive properties for live-cell imaging
Abstract
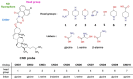
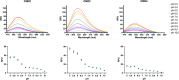
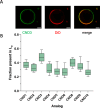
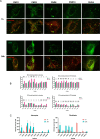
We present novel fluorescent cholesteryl probes (CNDs) with a modular design based on the solvatochromic 1,8-phthalimide scaffold. We have explored how different modules-linkers and head groups-affect the ability of these probes to integrate into lipid membranes and how they distribute intracellularly in mouse astrocytes and fibroblasts targeting lysosomes and lipid droplets. Each compound was assessed for its solvatochromic behavior in organic solvents and model membranes. Molecular dynamics simulations and lipid partitioning using giant unilamellar vesicles showed how these analogs behave in model membranes compared to cholesterol. Live-cell imaging demonstrated distinct staining patterns and cellular uptake behaviors, further validating the utility of these probes in biological systems. We compared the empirical results with those of BODIPY-cholesterol, a well-regarded fluorescent cholesterol analog. The internalization efficiency of fluorescent CND probes varies in different cell types and is affected mainly by the head groups. Our results demonstrate that the modular design significantly simplifies the creation of fluorescent cholesteryl probes bearing distinct spectral, biophysical, and cellular targeting features. It is a valuable toolkit for imaging in live cells, measuring cellular membrane dynamics, and studying cholesterol-related processes.
Keywords: Cholesterol; Fluorescence microscopy; Fluorescent reporter; Lipid droplets; Lipid rafts; Lysosomes.; Membrane; pH-sensitivity.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Modular Fluorescent Cholesterol Naphthalimide Probes And Their Application For Cholesterol Trafficking Studies In Cells.bioRxiv [Preprint]. 2024 Jun 25:2024.06.24.600118. doi: 10.1101/2024.06.24.600118. bioRxiv. 2024. PMID: 38979187 Free PMC article. Preprint.
-
Fluorescent Probes for Lipid Membranes: From the Cell Surface to Organelles.Acc Chem Res. 2023 Jan 3;56(1):1-12. doi: 10.1021/acs.accounts.2c00586. Epub 2022 Dec 19. Acc Chem Res. 2023. PMID: 36533992
-
Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications.Acc Chem Res. 2017 Feb 21;50(2):366-375. doi: 10.1021/acs.accounts.6b00517. Epub 2017 Jan 9. Acc Chem Res. 2017. PMID: 28067047
-
Emerging solvatochromic push-pull dyes for monitoring the lipid order of biomembranes in live cells.J Biochem. 2021 Oct 11;170(2):163-174. doi: 10.1093/jb/mvab078. J Biochem. 2021. PMID: 34213537 Review.
-
Potential of BODIPY-cholesterol for analysis of cholesterol transport and diffusion in living cells.Chem Phys Lipids. 2016 Jan;194:12-28. doi: 10.1016/j.chemphyslip.2015.08.007. Epub 2015 Aug 17. Chem Phys Lipids. 2016. PMID: 26291493 Review.
References
-
- Menon, A. K. Sterol gradients in cells. Curr. Opin. Cell. Biol.53, 37–43 (2018). - PubMed
-
- Simons, K. & Ikonen, E. Functional rafts in cell membranes. Nature387, 569–572 (1997). - PubMed
-
- Lev, S. Non-vesicular lipid transport by lipid-transfer proteins and beyond. Nat. Rev. Mol. Cell. Biol.11, 739–750 (2010). - PubMed
-
- Hao, M. et al. Vesicular and non-vesicular sterol transport in living cells: The endocytic recycling compartment is a major sterol storage organelle. J. Biol. Chem.277, 609–617 (2002). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

