Interruption of the visual cycle in a novel animal model induces progressive vision loss resembling Stargardts Disease
- PMID: 39730605
- PMCID: PMC11680818
- DOI: 10.1038/s41598-024-81869-y
Interruption of the visual cycle in a novel animal model induces progressive vision loss resembling Stargardts Disease
Abstract
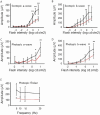
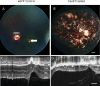
Mutations in the gene ABCA4 coding for photoreceptor-specific ATP-binding cassette subfamily A member 4, are responsible for Stargardts Disease type 1 (STGD1), the most common form of inherited macular degeneration. STGD1 typically declares early in life and leads to severe visual handicap. Abca4 gene-deletion mouse models of STGD1 accumulate lipofuscin, a hallmark of the disease, but unlike the human disease show no or only moderate structural changes and no functional decline. The human macula is highly enriched in cones, and reasoning that the low cone percentage in mice retinas (< 3%) might compromise faithful modelling of human maculopathies, we performed sub-retinal injections of CRISPR/Cas9-abca4 Adeno-Associated Virus constructs into young Sand Rats (Psammomys obesus), a diurnal rodent containing > 30% cones. Compared to control injections of AAV-abca4-GFP, treated eyes exhibited extensive retinal degeneration by two months. Sanger sequencing of the CRISPR targeted sequence show a clear edition of Abca4 gene. Non-invasive fundus imaging showed widespread photoreceptor loss, confirmed by ocular coherence tomography. Functional recording by single flash and flicker electroretinography showed significant decline in photopic (cone) light responses. Post-mortem real-time PCR, immunohistochemistry and western blotting showed significant decrease of cone-specific (MW cone opsin) but not rod-specific (rhodopsin) markers. Transmission electron microscopy showed large numbers of lipid inclusions in treated but not control retinal pigmented epithelium. Finally, ultra-high performance liquid chromatography analysis of whole P. obesus eyes showed the presence of all-trans retinal-dimer, not detected in rod-rich rat eyes. In conclusion, Abca4 knockout in P. obesus results in a predominantly cone degeneration phenotype, more accurately reflecting the etiology of human STGD1, and should be valuable for characterizing pathogenic pathways and exploring treatment options.
Keywords: Animal model; Cone photoreceptors; Electrophysiology; Gene editing; Retinal degeneration; Stargardt’s disease.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures







Similar articles
-
Stargardt's Disease: Molecular Pathogenesis and Current Therapeutic Landscape.Int J Mol Sci. 2025 Jul 21;26(14):7006. doi: 10.3390/ijms26147006. Int J Mol Sci. 2025. PMID: 40725253 Free PMC article. Review.
-
Increased cone sensitivity to ABCA4 deficiency provides insight into macular vision loss in Stargardt's dystrophy.Biochim Biophys Acta. 2012 Jul;1822(7):1169-79. doi: 10.1016/j.bbadis.2011.10.007. Epub 2011 Oct 13. Biochim Biophys Acta. 2012. PMID: 22033104 Free PMC article.
-
A new mouse model for PRPH2 pattern dystrophy exhibits functional compensation prior and subsequent to retinal degeneration.Hum Mol Genet. 2024 Nov 5;33(21):1916-1928. doi: 10.1093/hmg/ddae128. Hum Mol Genet. 2024. PMID: 39231530 Free PMC article.
-
Extremely hypomorphic and severe deep intronic variants in the ABCA4 locus result in varying Stargardt disease phenotypes.Cold Spring Harb Mol Case Stud. 2018 Aug 1;4(4):a002733. doi: 10.1101/mcs.a002733. Print 2018 Aug. Cold Spring Harb Mol Case Stud. 2018. PMID: 29848554 Free PMC article.
-
Genotype-phenotype correlation in a German family with a novel complex CRX mutation extending the open reading frame.Ophthalmology. 2007 Jul;114(7):1348-1357.e1. doi: 10.1016/j.ophtha.2006.10.034. Epub 2007 Feb 22. Ophthalmology. 2007. PMID: 17320181
Cited by
-
Investigation of ABCA4 Missense Variants and Potential Small Molecule Rescue in Retinal Organoids.Invest Ophthalmol Vis Sci. 2025 Jul 1;66(9):58. doi: 10.1167/iovs.66.9.58. Invest Ophthalmol Vis Sci. 2025. PMID: 40693713 Free PMC article.
-
Stargardt's Disease: Molecular Pathogenesis and Current Therapeutic Landscape.Int J Mol Sci. 2025 Jul 21;26(14):7006. doi: 10.3390/ijms26147006. Int J Mol Sci. 2025. PMID: 40725253 Free PMC article. Review.
References
-
- Baylor, D. A. Photoreceptor signals and vision. Investig. Ophthalmol. Vis. Sci.2, 34–49 (1987). - PubMed
-
- Allikmets, R. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat. Genet.17, 122 (1997). - PubMed
-
- Rotenstreich, Y., Fishman, G. A. & Anderson, R. J. Visual acuity loss and clinical observations in a large series of patients with Stargardt disease. Ophthalmology110, 1151–1158 (2003). - PubMed
-
- Fujinami, K. et al. A longitudinal study of Stargardt disease: clinical and electrophysiologic assessment, progression, and genotype correlations. Am. J. Ophthalmol.155, 1075–1088 (2013). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

