New autoimmune disorder development after immune reconstitution therapy for multiple sclerosis
- PMID: 39730657
- PMCID: PMC11681027
- DOI: 10.1038/s41598-024-82196-y
New autoimmune disorder development after immune reconstitution therapy for multiple sclerosis
Abstract
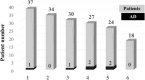
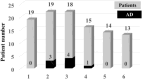
Immune reconstitution therapy (IRT) is a relatively new and highly effective treatment option for multiple sclerosis (MS). Uncertainty regarding the development of autoimmune disorders (ADs) after some therapies remains. The aim of this study was to assess new AD development after IRT in MS patients and to describe the nature of those ADs and the time to onset. A total of 179 patients with relapsing multiple sclerosis (37 after autologous haematopoietic stem cell transplantation (AHSCT), 19 after alemtuzumab (ALE) and 123 after cladribine (CLA) treatment) over a ten year period were included in the study. ADs were observed in 6 patients (16.2%) after AHSCT, 8 patients (42.1%) after ALE and 2 patients (1.6%) after CLA treatment. ADs developed earlier after ALE infusions, but later after AHSCT except for cytopenias. Neurologists should be attentive to the development of secondary ADs after ALE and AHSCT in MS patients.
Keywords: Alemtuzumab; Autoimmune disorder; Autologous haematopoietic stem cell transplantation; Cladribine; Immune reconstitution therapy; Relapsing multiple sclerosis.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
-
- Lemtrada product information. European Medicines Agency. 2023. https://www.ema.europa.eu/en/documents/product-information/lemtrada-epar... Accessed on 06. June, 2024.
-
- Mavenclad product information. European Medicines Agency. https://www.ema.europa.eu/en/documents/product-information/mavenclad-epa... Accessed on 06. June, 2024.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

