Transduction of jellyfish superoxide dismutase mediated by TAT peptide ameliorates H2O2-induced oxidative stress in HaCaT cells
- PMID: 39730660
- PMCID: PMC11680928
- DOI: 10.1038/s41598-024-82261-6
Transduction of jellyfish superoxide dismutase mediated by TAT peptide ameliorates H2O2-induced oxidative stress in HaCaT cells
Abstract
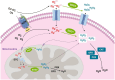
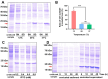
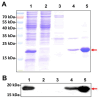
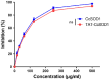
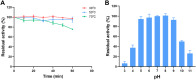
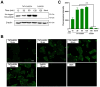
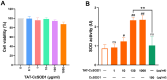
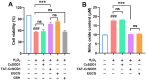
Superoxide dismutase (SOD) plays important roles in the balance of oxidation and antioxidation in body mostly by scavenging superoxide anion free radicals (O2.-). Previously, we reported a novel Cu/Zn SOD from jellyfish Cyanea capillata, named CcSOD1, which exhibited excellent SOD activity and high stability. TAT peptide is a common type of cell penetrating peptides (CPPs) that efficiently deliver extracellular biomacromolecules into cytoplasm. In this study, we constructed a recombinant expression vector that combined the coding sequences of TAT peptide and CcSOD1, and then obtained sufficient and high-purity TAT-CcSOD1 fusion protein. Compared with some reported SODs/CPP-SODs, TAT-CcSOD1 possessed stronger tolerance to heat and acid-base environment. TAT-CcSOD1 efficiently penetrated cell membrane and significantly enhanced the O2.- scavenging ability in cells, and attenuated H2O2-induced cytotoxicity and NO generation in HaCaT cells. This study serves as a critical step forward for the application of TAT-CcSOD1 as a potential protective/therapeutic agent against oxidative stress-related conditions in the future.
Keywords: Antioxidant; Oxidative stress; Superoxide dismutase; TAT peptide; Transmembrane.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

