Neratinib enhances the efficacy of CDK4/6 inhibitor plus endocrine therapy in HR+/HER2-low breast cancer cell line ZR-75-1 via hsa-miR-23a-5p
- PMID: 39730704
- PMCID: PMC11680982
- DOI: 10.1038/s41598-024-82137-9
Neratinib enhances the efficacy of CDK4/6 inhibitor plus endocrine therapy in HR+/HER2-low breast cancer cell line ZR-75-1 via hsa-miR-23a-5p
Abstract
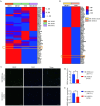
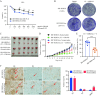
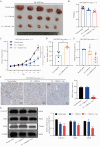
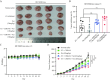
HR+/HER2-low breast cancer is a significant subgroup of conventional HR+/HER2-negative breast cancer, and combination of CDK4/6 inhibitor and endocrine therapy is the standard first-line and second-line treatments for advanced HR+/HER2-low breast cancer. Nevertheless, it remains uncertain whether HER2 signaling affects the effectiveness of CDK4/6 inhibitor administered in combination with endocrine therapy for HR+/HER2-low breast cancer and suitable intervention measures. This study revealed poor efficacy for CDK4/6 inhibitor combined with endocrine therapy for HR+/HER2-low breast cancer in vitro and in vivo models. Secondly, suppression of HER2 gene expression in HR+/HER2-low breast cancer cells resulted in significantly improved efficacy for CDK4/6 inhibitor combined with endocrine therapy. Furthermore, the anti-HER inhibitor neratinib was administered to enhance the effectiveness of CDK4/6 inhibitor combined with endocrine therapy in HR+/HER2-low breast cancer by inhibiting the HER2 pathway and lowering HER2 mRNA expression. Strikingly, neratinib reversed the efficacy of CDK4/6 inhibitor and endocrine therapy by reducing HER2 mRNA stability in HR+/HER2-low breast cancer through the interaction of HER2 3'-UTR region with hsa-miR-23a-5p. Even after reducing neratinib dosage to the standard 1/2 dose (20 mg/kg), it remained highly effective and well-tolerated. This study provides a viable and well-tolerated triple combination therapy for clinical HR+/HER2-low breast cancer.
Keywords: CDK4/6 inhibitor; Endocrine therapy; HER2 mRNA stability; HR+/HER2-low; Hsa-miR-23a-5p; Neratinib.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: This study was conducted according to ARRIVE guidelines 2.0. Liushan Chen, Lingling Ye, and Yuqi Liang are co-first authors. Yingchao Wu, Qianqian Guo, and Qianjun Chen are co-corresponding authors.
Figures



Similar articles
-
Combining Neratinib with CDK4/6, mTOR, and MEK Inhibitors in Models of HER2-positive Cancer.Clin Cancer Res. 2021 Mar 15;27(6):1681-1694. doi: 10.1158/1078-0432.CCR-20-3017. Epub 2021 Jan 7. Clin Cancer Res. 2021. PMID: 33414137 Free PMC article.
-
EGFR and HER2 hyper-activation mediates resistance to endocrine therapy and CDK4/6 inhibitors in ER+ breast cancer.Cancer Lett. 2024 Jul 1;593:216968. doi: 10.1016/j.canlet.2024.216968. Epub 2024 May 23. Cancer Lett. 2024. PMID: 38788968
-
Clinical efficacy of CDK4/6 inhibitor plus endocrine therapy in HR-positive/HER2-0 and HER2-low-positive metastatic breast cancer: a secondary analysis of PALOMA-2 and PALOMA-3 trials.EBioMedicine. 2024 Jul;105:105186. doi: 10.1016/j.ebiom.2024.105186. Epub 2024 Jun 10. EBioMedicine. 2024. PMID: 38861871 Free PMC article. Clinical Trial.
-
Cyclin-dependent kinase 4 and 6 inhibitors in hormone receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer: a meta-analysis of randomized clinical trials.Breast Cancer Res Treat. 2020 Feb;180(1):21-32. doi: 10.1007/s10549-020-05528-2. Epub 2020 Jan 22. Breast Cancer Res Treat. 2020. PMID: 31970560 Review.
-
Abemaciclib: a CDK4/6 inhibitor for the treatment of HR+/HER2- advanced breast cancer.Drug Des Devel Ther. 2018 Feb 16;12:321-330. doi: 10.2147/DDDT.S137783. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 29497278 Free PMC article. Review.
References
-
- Giuliano, M. et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol.20 (10), 1360–1369 (2019). - PubMed
-
- Hortobagyi, G. N. et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol.29 (7), 1541–1547 (2018). - PubMed
MeSH terms
Substances
Grants and funding
- KC2022-ZZ-0091-1/Science and Technology Innovation Medical Development Foundation of Beijing
- KC2021-ZZ-0010-9/Science and Technology Innovation Medical Development Foundation of Beijing
- 2022A1515110859/Basic and Applied Basic Research Foundation of Guangdong Province
- 82274513/National Natural Science Foundation of China
- HQL2024PZ023/Project supported by the Incubation Program for the Science and Technology Development of Chinese Medicine Guangdong Laboratory
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

