Generation of a selective senolytic platform using a micelle-encapsulated Sudan Black B conjugated analog
- PMID: 39730824
- PMCID: PMC11754095
- DOI: 10.1038/s43587-024-00747-4
Generation of a selective senolytic platform using a micelle-encapsulated Sudan Black B conjugated analog
Erratum in
-
Author Correction: Generation of a selective senolytic platform using a micelle-encapsulated Sudan Black B conjugated analog.Nat Aging. 2025 Mar;5(3):528. doi: 10.1038/s43587-025-00820-6. Nat Aging. 2025. PMID: 39890937 Free PMC article. No abstract available.
Abstract
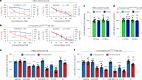
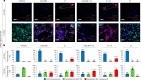
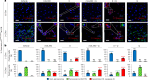
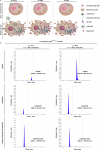
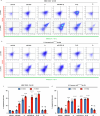
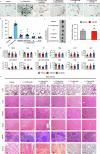
The emerging field of senolytics is centered on eliminating senescent cells to block their contribution to the progression of age-related diseases, including cancer, and to facilitate healthy aging. Enhancing the selectivity of senolytic treatments toward senescent cells stands to reduce the adverse effects associated with existing senolytic interventions. Taking advantage of lipofuscin accumulation in senescent cells, we describe here the development of a highly efficient senolytic platform consisting of a lipofuscin-binding domain scaffold, which can be conjugated with a senolytic drug via an ester bond. As a proof of concept, we present the generation of GL392, a senolytic compound that carries a dasatinib senolytic moiety. Encapsulation of the GL392 compound in a micelle nanocarrier (termed mGL392) allows for both in vitro and in vivo (in mice) selective elimination of senescent cells via targeted release of the senolytic agent with minimal systemic toxicity. Our findings suggest that this platform could be used to enhance targeting of senotherapeutics toward senescent cells.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: Described compounds are in patent pending status: (1) Greek Patent Application No. 20240100309, (2) UK Patent Application No. GB2406749.8 and (3) international Patent Cooperation Treaty filing. Patent competing interests concern N.K. and V.G.G. only. The other authors declare no competing interests.
Figures






Similar articles
-
A novel platform for precise senolysis.Mech Ageing Dev. 2025 Jun;225:112056. doi: 10.1016/j.mad.2025.112056. Epub 2025 Apr 6. Mech Ageing Dev. 2025. PMID: 40199385
-
Senolytic Prodrugs: A Promising Approach to Enhancing Senescence-Targeting Intervention.Chembiochem. 2024 Nov 18;25(22):e202400355. doi: 10.1002/cbic.202400355. Epub 2024 Sep 18. Chembiochem. 2024. PMID: 39058554 Review.
-
Chemical Strategies for the Detection and Elimination of Senescent Cells.Acc Chem Res. 2024 May 7;57(9):1238-1253. doi: 10.1021/acs.accounts.3c00794. Epub 2024 Apr 11. Acc Chem Res. 2024. PMID: 38604701 Free PMC article. Review.
-
Molecular modelling of the FOXO4-TP53 interaction to design senolytic peptides for the elimination of senescent cancer cells.EBioMedicine. 2021 Nov;73:103646. doi: 10.1016/j.ebiom.2021.103646. Epub 2021 Oct 21. EBioMedicine. 2021. PMID: 34689087 Free PMC article.
-
Senolytic drugs dasatinib and quercetin combined with Carboplatin or Olaparib reduced the peritoneal and adipose tissue metastasis of ovarian cancer.Biomed Pharmacother. 2024 May;174:116474. doi: 10.1016/j.biopha.2024.116474. Epub 2024 Mar 21. Biomed Pharmacother. 2024. PMID: 38518604
Cited by
-
Rejuvenating the immune system.Mol Oncol. 2025 Mar;19(3):584-587. doi: 10.1002/1878-0261.13802. Epub 2025 Jan 13. Mol Oncol. 2025. PMID: 39804992 Free PMC article.
-
Molecular Regulation of SASP in Cellular Senescence: Therapeutic Implications and Translational Challenges.Cells. 2025 Jun 20;14(13):942. doi: 10.3390/cells14130942. Cells. 2025. PMID: 40643463 Free PMC article. Review.
-
Cellular senescence in cancer: from mechanism paradoxes to precision therapeutics.Mol Cancer. 2025 Aug 8;24(1):213. doi: 10.1186/s12943-025-02419-2. Mol Cancer. 2025. PMID: 40781676 Free PMC article. Review.
-
HPV-Negative Oral Squamous Cell Carcinoma Arising from Oral Submucous Fibrosis with p16INK4A Positivity and Cellular Senescence: A Case Report.J Clin Exp Dent. 2025 Apr 1;17(4):e479-e482. doi: 10.4317/jced.62698. eCollection 2025 Apr. J Clin Exp Dent. 2025. PMID: 40375838 Free PMC article.
-
Regulation of cellular senescence in tumor progression and therapeutic targeting: mechanisms and pathways.Mol Cancer. 2025 Apr 2;24(1):106. doi: 10.1186/s12943-025-02284-z. Mol Cancer. 2025. PMID: 40170077 Free PMC article. Review.
References
-
- Gorgoulis, V. et al. Cellular senescence: defining a path forward. Cell31, 813–827 (2019). - PubMed
-
- Zampetidis, C. P. et al. A recurrent chromosomal inversion suffices for driving escape from oncogene-induced senescence via subTAD reorganization. Mol. Cell81, 4907–4923 (2021). - PubMed
-
- Bartkova, J. et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature444, 633–637 (2006). - PubMed
-
- Halazonetis, T. D., Gorgoulis, V. G. & Bartek, J. An oncogene-induced DNA damage model for cancer development. Science319, 1352–1355 (2008). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

