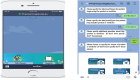
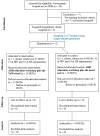
Effectiveness of the modified TaWai mobile application for reporting adverse drug reaction in Lao PDR: a cluster randomized controlled trial
- PMID: 39730897
- PMCID: PMC11681246
- DOI: 10.1038/s41598-024-82474-9
Effectiveness of the modified TaWai mobile application for reporting adverse drug reaction in Lao PDR: a cluster randomized controlled trial
Abstract
Spontaneous adverse drug reactions (ADRs) reporting by health care professionals (HCPs) plays a vital role in pharmacovigilance (PV). However, under-reporting remain a major challenge worldwide, especially in low and middle-income countries, including Lao PDR. This cluster-randomized controlled trial evaluated the effectiveness of the modified TaWai mobile app for ADR reporting compared with the usual practice in hospitals. Two tertiary hospitals in Lao PDR (cluster units) were randomized into two groups: the intervention group (16 HCPs), which used the modified TaWai mobile app along with an educational workshop, and the control group (18 HCPs), which followed usual practice with the same educational workshop. The intervention group reported more ADR cases (28 vs. 3), and produced a higher number of high-quality reports (28 vs. 2) than the control group. The modified TaWai mobile app was highly rated by all participating HCPs. Questionnaire responses indicated that the tool is user-friendly, time-efficient, and well-suited for ADR reporting in hospitals in Lao PDR. In conclusion, these findings highlight the potential of the modified TaWai mobile app to enhance ADR reporting practices in hospitals, and its features make it a promising solution for strengthening PV in Lao PDR and similar settings.
Keywords: ADR reporting; Lao PDR; Low/middle-income countries; Pharmacovigilance; TaWai mobile App.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Systematic review and meta-analysis on effectiveness of strategies for enhancing adverse drug reaction reporting.J Am Pharm Assoc (2003). 2025 Jan-Feb;65(1):102293. doi: 10.1016/j.japh.2024.102293. Epub 2024 Nov 9. J Am Pharm Assoc (2003). 2025. PMID: 39522823
-
Dedicated mobile application for drug adverse reaction reporting by patients with relapsing remitting multiple sclerosis (Vigip-SEP study): study protocol for a randomized controlled trial.Trials. 2018 Mar 9;19(1):174. doi: 10.1186/s13063-018-2560-4. Trials. 2018. PMID: 29523169 Free PMC article.
-
An exploratory study of knowledge, attitudes, practice and barriers towards adverse drug reaction reporting among healthcare professionals in Malta.Int J Risk Saf Med. 2024;35(3):271-286. doi: 10.3233/JRS-230055. Int J Risk Saf Med. 2024. PMID: 38820023
-
Evaluation of patient reporting of adverse drug reactions to the UK 'Yellow Card Scheme': literature review, descriptive and qualitative analyses, and questionnaire surveys.Health Technol Assess. 2011 May;15(20):1-234, iii-iv. doi: 10.3310/hta15200. Health Technol Assess. 2011. PMID: 21545758 Review.
-
Facilitators and Barriers to Uptake of the Med Safety Mobile App for Adverse Drug Reaction Reporting by Health Workers in Uganda: A Qualitative Study.Drug Saf. 2023 Jun;46(6):565-574. doi: 10.1007/s40264-023-01303-6. Epub 2023 Apr 25. Drug Saf. 2023. PMID: 37097426 Free PMC article.
References
-
- World Health Organization. The importance of pharmacovigilance. Safety Monitoring of Medicinal Products. https://iris.who.int/handle/10665/42493 (2002).
-
- Gahr, M., Eller, J., Connemann, B. J. & Schönfeldt-Lecuona, C. Underreporting of adverse drug reactions: Results from a survey among physicians. Eur. Psychiatry41, S369–S369. 10.1016/j.eurpsy.2017.02.377 (2020).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials