Updated imaging markers in cerebral amyloid angiopathy: What radiologists need to know
- PMID: 39730931
- PMCID: PMC12053366
- DOI: 10.1007/s11604-024-01720-2
Updated imaging markers in cerebral amyloid angiopathy: What radiologists need to know
Abstract
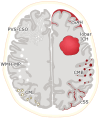
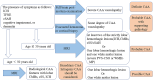
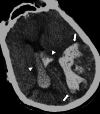
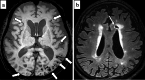
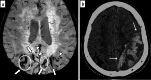
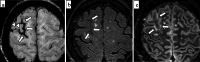
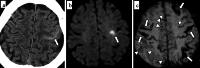
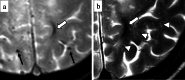
Cerebral amyloid angiopathy (CAA) is an age-related small vessel disease pathologically characterized by the progressive accumulation of amyloid-beta (Aβ) peptide in cerebrovascular walls, affecting both cortical and leptomeningeal vessels. Amyloid deposition results in fragile vessels, which may lead to lobar intracerebral hemorrhage (ICH) and cognitive impairment. To evaluate the probability and severity of CAA, the imaging markers depicted on CT and MRI techniques are crucial, as brain pathological examination is highly invasive. Although the Boston criteria have established diagnostic value and have been updated to version 2.0, due to an aging population, the patients with CAA should also be assessed for their risk of future ICH or cognitive impairment. Furthermore, an increased awareness of CAA is essential when introducing anticoagulants for infarct in elderly patients or anti-amyloid antibodies for Alzheimer's disease, as these may worsen CAA-related hemorrhagic lesions. However, the radiological literature on CAA has not been comprehensively updated. Here, we review the imaging markers of CAA and clinical significance. We also discuss the clinical and imaging characteristics of CAA-related inflammation, amyloid-related imaging abnormalities, and iatrogenic-CAA.
Keywords: Alzheimer’s disease; Cerebral amyloid angiopathy; MRI; Small vessel disease.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: There is no conflict of interest. Ethical approval: Not applicable for review article. Informed consent: Not applicable for review article.
Figures
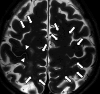
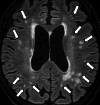
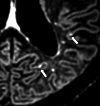
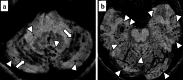
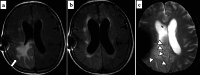
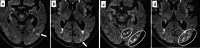
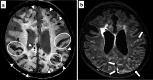
Similar articles
-
The Boston criteria version 2.0 for cerebral amyloid angiopathy: a multicentre, retrospective, MRI-neuropathology diagnostic accuracy study.Lancet Neurol. 2022 Aug;21(8):714-725. doi: 10.1016/S1474-4422(22)00208-3. Lancet Neurol. 2022. PMID: 35841910 Free PMC article.
-
MRI and CT imaging biomarkers of cerebral amyloid angiopathy in lobar intracerebral hemorrhage.Int J Stroke. 2023 Jan;18(1):85-94. doi: 10.1177/17474930211062478. Epub 2022 Jan 7. Int J Stroke. 2023. PMID: 34994246
-
Validation of the Boston Criteria Version 2.0 for Cerebral Amyloid Angiopathy in Patients Presenting With Intracerebral Hemorrhage.Neurology. 2025 Apr 8;104(7):e213460. doi: 10.1212/WNL.0000000000213460. Epub 2025 Mar 3. Neurology. 2025. PMID: 40030097
-
Neuroradiological Findings in Cerebral Amyloid Angiopathy with a Particular Consideration of the Boston Criteria 2.0: An Imaging Review.Biomolecules. 2024 Nov 17;14(11):1459. doi: 10.3390/biom14111459. Biomolecules. 2024. PMID: 39595634 Free PMC article. Review.
-
Amyloid positron emission tomography in sporadic cerebral amyloid angiopathy: A systematic critical update.Neuroimage Clin. 2017 May 5;15:247-263. doi: 10.1016/j.nicl.2017.05.002. eCollection 2017. Neuroimage Clin. 2017. PMID: 28560150 Free PMC article. Review.
Cited by
-
Iatrogenic Cerebral Amyloid Angiopathy After Childhood Brain Surgery: Novel Findings of MRI and CT.Neurol Int. 2025 Apr 24;17(5):64. doi: 10.3390/neurolint17050064. Neurol Int. 2025. PMID: 40423220 Free PMC article.
-
Non-Invasive Retinal Biomarkers for Early Diagnosis of Alzheimer's Disease.Biomedicines. 2025 Jan 24;13(2):283. doi: 10.3390/biomedicines13020283. Biomedicines. 2025. PMID: 40002697 Free PMC article. Review.
-
Probable Cerebral Amyloid Angiopathy-Related Inflammation Presenting as an Incidental MRI Finding in an Elderly Patient: A Case Report.Cureus. 2025 May 17;17(5):e84282. doi: 10.7759/cureus.84282. eCollection 2025 May. Cureus. 2025. PMID: 40524983 Free PMC article.
References
-
- Thal DR, Ghebremedhin E, Rüb U, Yamaguchi H, Del Tredici K, Braak H. Two types of sporadic cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2002;61(3):282–93. - PubMed
-
- Attems J. Sporadic cerebral amyloid angiopathy: pathology, clinical implications, and possible pathomechanisms. Acta Neuropathol. 2005;110(4):345–59. - PubMed
-
- Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry. 2012;83(2):124–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

