The interplay of factors in metabolic syndrome: understanding its roots and complexity
- PMID: 39731011
- PMCID: PMC11673706
- DOI: 10.1186/s10020-024-01019-y
The interplay of factors in metabolic syndrome: understanding its roots and complexity
Abstract
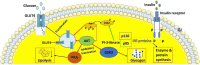
Metabolic syndrome (MetS) is an indicator and diverse endocrine syndrome that combines different metabolic defects with clinical, physiological, biochemical, and metabolic factors. Obesity, visceral adiposity and abdominal obesity, dyslipidemia, insulin resistance (IR), elevated blood pressure, endothelial dysfunction, and acute or chronic inflammation are the risk factors associated with MetS. Abdominal obesity, a hallmark of MetS, highlights dysfunctional fat tissue and increased risk for cardiovascular disease and diabetes. Insulin, a vital peptide hormone, regulates glucose metabolism throughout the body. When cells become resistant to insulin's effects, it disrupts various molecular pathways, leading to IR. This condition is linked to a range of disorders, including obesity, diabetes, fatty liver disease, cardiovascular disease, and polycystic ovary syndrome. Atherogenic dyslipidemia is characterized by three key factors: high levels of small, low-dense lipoprotein (LDL) particles and triglycerides, alongside low levels of high-density lipoprotein (HDL), the "good" cholesterol. Such a combination is a major player in MetS, where IR is a driving force. Atherogenic dyslipidemia contributes significantly to the development of atherosclerosis, which can lead to cardiovascular disease. On top of that, genetic alteration and lifestyle factors such as diet and exercise influence the complexity and progression of MetS. To enhance our understanding and consciousness, it is essential to understand the fundamental pathogenesis of MetS. This review highlights current advancements in MetS research including the involvement of gut microbiome, epigenetic regulation, and metabolomic profiling for early detection of Mets. In addition, this review emphasized the epidemiology and fundamental pathogenesis of MetS, various risk factors, and their preventive measures. The goal of this effort is to deepen understanding of MetS and encourage further research to develop effective strategies for preventing and managing complex metabolic diseases.
Keywords: Dyslipidemia; Epidemiology; Insulin resistance; Metabolic syndrome; Obesity; Pathogenesis.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabetic Med. 2006;23(5):469–80. - PubMed
-
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

