Dapagliflozin in acute heart failure management: a systematic review and meta-analysis of safety and effectiveness
- PMID: 39731023
- PMCID: PMC11681638
- DOI: 10.1186/s12872-024-04412-x
Dapagliflozin in acute heart failure management: a systematic review and meta-analysis of safety and effectiveness
Abstract
Background: Acute Heart Failure (AHF) presents as a serious pathophysiological disease with significant morbidity and mortality rates, requiring immediate medical intervention. Traditional treatment involves diuretics and vasodilators, but a subset of patients develop resistance due to acute cardiorenal syndrome. Dapagliflozin, categorized as a sodium-glucose cotransporter-2 inhibitor (SGLT2i), has emerged as a promising therapy for AHF, demonstrating substantial benefits in reducing both mortality and morbidity among patients. The purpose of this meta-analysis and systematic review is to determine dapagliflozin's safety and efficacy in AHF patients.
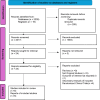
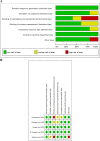
Methods: In accordance with PRISMA guidelines, we conducted a systematic search across several databases (PubMed, Science Direct, and Cochrane Library) up to June 2024 to identify randomized controlled trials (RCTs) that compared dapagliflozin with control treatments in patients with AHF. Key outcomes of interest included In-Hospital Cardiovascular mortality rates, duration of hospitalization, and instances of in-hospital worsening. Data extraction and quality assessment adhered to established protocols and the results were evaluated using Review Manager (RevMan Version 5.4.1) The assessment of bias risk follows the principles established in the Cochrane Handbook for Systematic Reviews and Meta-Analysis.
Results: Five RCTs comprising 912 patients met the inclusion criteria. Dapagliflozin significantly reduced In-Hospital Cardiovascular mortality (RR 0.56, 95% CI 0.36-0.88, p = 0.01, I²=26%) and 30-day hospital readmissions (RR 0.73, CI 0.54-0.99, p = 0.05, I²=7%). However, dapagliflozin did not significantly affect the length of hospital stay (MD -0.11, CI -0.73-0.51, p = 0.72, I²=60%) or the incidence of hypotension (RR 0.82, CI 0.36-1.84, p = 0.63, I²=0%). A significant weight change was observed (MD 0.93, CI 0.03-1.83, p = 0.04, I²=95%), which was resolved upon sensitivity analysis (MD 1.34, CI 1.02-1.66, p < 0.0001, I²=0%). No significant effects were found for worsening renal failure or changes in GFR in this study.
Conclusion: Dapagliflozin appears to be beneficial in reducing In-Hospital Cardiovascular mortality and 30-day hospital readmissions in AHF patients. Although it demonstrates potential, additional research is needed to establish its significance in AHF management. Further investigation with larger sample sizes, different doses, and comprehensive safety and cost-effectiveness is imperative to thoroughly evaluate the safety and clinical efficacy of Dapagliflozin, underscoring the necessity for additional data to substantiate its role in managing patients with AHF.
Clinical trial number: Not applicable.
Keywords: Acute heart failure; Dapagliflozin; In-hospital cardiovascular mortality; Meta analysis; SGLT2i.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Consent to participate: Not applicable. Consent to publish: Not applicable. Human ethics and consent to participate approval: Not applicable. Competing interests: The authors declare no competing interests.
Figures
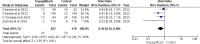
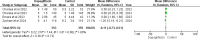
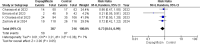
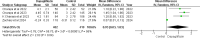
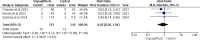

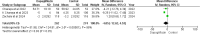
Similar articles
-
Efficacy and safety of dapagliflozin in acute heart failure: Rationale and design of the DICTATE-AHF trial.Am Heart J. 2021 Feb;232:116-124. doi: 10.1016/j.ahj.2020.10.071. Epub 2020 Nov 2. Am Heart J. 2021. PMID: 33144086
-
Efficacy and safety of empagliflozin in acute heart failure: a systematic review and meta-analysis.Future Cardiol. 2025 Jun;21(7):495-501. doi: 10.1080/14796678.2025.2499374. Epub 2025 May 5. Future Cardiol. 2025. PMID: 40324865 Review.
-
Dapagliflozin in Heart Failure: A Comprehensive Meta-analysis on Functional Capacity, Symptoms, and Safety Outcomes.Am J Cardiovasc Drugs. 2024 Nov;24(6):753-773. doi: 10.1007/s40256-024-00669-x. Epub 2024 Sep 11. Am J Cardiovasc Drugs. 2024. PMID: 39261443
-
Effect of Dapagliflozin on Heart Failure and Mortality in Type 2 Diabetes Mellitus.Circulation. 2019 May 28;139(22):2528-2536. doi: 10.1161/CIRCULATIONAHA.119.040130. Epub 2019 Mar 18. Circulation. 2019. PMID: 30882238 Clinical Trial.
-
Efficacy and safety of sodium-glucose cotransporter 2 inhibitors initiation in patients with acute heart failure, with and without type 2 diabetes: a systematic review and meta-analysis.Cardiovasc Diabetol. 2022 Feb 5;21(1):20. doi: 10.1186/s12933-022-01455-2. Cardiovasc Diabetol. 2022. PMID: 35123480 Free PMC article.
Cited by
-
A meta-analytic review of the safety and efficacy of semaglutide in type 2 diabetes mellitus and chronic kidney disease patients.Ann Med Surg (Lond). 2025 Mar 27;87(4):2278-2285. doi: 10.1097/MS9.0000000000003126. eCollection 2025 Apr. Ann Med Surg (Lond). 2025. PMID: 40212218 Free PMC article.
-
The impact of sodium-glucose co-transporter-2 inhibitors on serum sodium and potassium in patients with Heart Failure: a systematic review and meta-analysis.BMC Cardiovasc Disord. 2025 Apr 3;25(1):252. doi: 10.1186/s12872-025-04704-w. BMC Cardiovasc Disord. 2025. PMID: 40181257 Free PMC article.
References
-
- Cosentino N, Campodonico J. Acute heart failure: diagnosis first and then treatment. Int J Cardiol. Oct. 2018;269:224–5. 10.1016/j.ijcard.2018.07.032. - PubMed
-
- Köksal F, Aldemir M, Levent F, Emren SV, Nazlı C. The Effect of the practical treatments for Acute Heart failure on In-Hospital Cardiovascular mortality: a real-world study. Kafkas J Med Sci. 2022;12(1):37–43. 10.5505/KJMS.2022.64426.
-
- Di Somma S, Magrini L. Drug Therapy for Acute Heart Failure. Revista Española de Cardiología (English Edition). 2015;68(8):706–13. 10.1016/J.REC.2015.02.019 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

