Assessment of the safety and gut microbiota modulation ability of an infant formula containing Bifidobacterium animalis ssp. lactis CP-9 or Lactobacillus salivarius AP-32 and the effects of the formula on infant growth outcomes: insights from a four-month clinical study in infants under two months old
- PMID: 39731060
- PMCID: PMC11674581
- DOI: 10.1186/s12887-024-05289-7
Assessment of the safety and gut microbiota modulation ability of an infant formula containing Bifidobacterium animalis ssp. lactis CP-9 or Lactobacillus salivarius AP-32 and the effects of the formula on infant growth outcomes: insights from a four-month clinical study in infants under two months old
Abstract
Background: Breast milk is a natural treasure for infants, and its microbiota contains a rich array of bacterial species. When breastfeeding is not possible, infant formula with probiotics can be used as a sole source or as a breast milk supplement. The main aim of this study was to evaluate the growth outcomes and tolerance of infants consuming an infant formula containing Bifidobacterium animalis ssp. lactis CP-9 (B. animalis CP-9) or Lactobacillus salivarius AP-32 (L. salivarius AP-32), which were isolated from breast milk and the guts of healthy infants. The safety of these strains in terms of antibiotic resistance and their ability to modulate the gut microbiota were also evaluated.
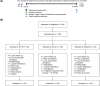
Methods: One hundred eighty healthy infants were included in this study and separated into three groups: the control group, the L. salivarius AP-32 group, and the B. animalis CP-9 group. In this clinical study, adverse events, growth effects, and the incidence of allergies and gastrointestinal disorders in infants consuming infant formula containing B. animalis CP-9 or L. salivarius AP-32 were evaluated. Finally, the impact of the probiotic infant formula on the gut microbiota was elucidated via next-generation sequencing (NGS) analysis.
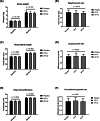
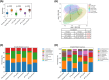
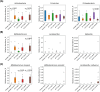
Results: The 4-month interventional study revealed that body weight, recumbent length, and head circumference were similar among the three groups. No adverse events related to the intervention were observed. The microbiota composition was more diverse on day 0 and became more uniform by month 4. B. animalis CP-9 and L. salivarius AP-32 were found to be susceptible to streptomycin, tetracycline, erythromycin, clindamycin, chloramphenicol, and ampicillin.
Conclusions: The use of infant formula containing B. animalis CP-9 and L. salivarius AP-32 was considered safe and well tolerated, with no adverse events observed during the study. While these strains showed low antibiotic resistance and no immediate concerns related to antibiotic resistance genes, further research is needed to comprehensively assess their long-term safety and efficacy and the potential risk of horizontal gene transfer in broader contexts.
Trial registration: The trial was registered with the US Library of Medicine (clinicaltrials.gov) with the number NCT03993301 on 20/06/2019.
Keywords: Gastrointestinal disorders; Infant formula; Infant growth; Probiotics.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study adhered to the guidelines of the Declaration of Helsinki, was approved by the China Medical University & Hospital Research Ethics Committee (IRB No. CMUH108-REC2-005), and was registered with the US Library of Medicine ( www.clinicaltrial.gov ) with the identifier NCT03993301. Written informed consent was obtained from the parents or legal guardians of all participants. Consent for publication: Not applicable. Competing interests: Glac Biotech Co., Ltd. provided financial support in the form of salaries for J. F. Chen, H.S. Wang, Y.Y. Huang, K.C. Hsia, J.H. Lin, Y.W. Kuo, C.M. Li, Y.C. Hsu, S.Y. Tsai, and H.H. Ho but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. S.P. Shen and H.C. Lin declare that they have no competing interests.
Figures

References
-
- American Academy of Pediatrics. American Academy of Pediatrics Work Group on Breastfeeding. Policy Statement: Breastfeeding and the use of human milk. Pediatrics. 1997;100(6):1035–9. - PubMed
-
- Additives EPo, Feed PoSuiA. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA Journal. 2012;10(6):2740.
-
- Braegger C, Chmielewska A, Decsi T, Kolacek S, Mihatsch W, Moreno L, Piescik M, Puntis J, Shamir R, Szajewska H. Supplementation of infant formula with probiotics and/or prebiotics: a systematic review and comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr. 2011;52(2):238–50. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

