Prognostic value of interim PET/CT in GCB and non-GCB DLBCL: comparison of the Deauville five-point scale and the ΔSUVmax method
- PMID: 39731077
- PMCID: PMC11681756
- DOI: 10.1186/s12885-024-13360-w
Prognostic value of interim PET/CT in GCB and non-GCB DLBCL: comparison of the Deauville five-point scale and the ΔSUVmax method
Abstract
Background: This study aimed to identify the prognostic value of interim 18F-FDG PET/CT (I-PET) for germinal center B-cell-like (GCB) and non-GCB diffuse large B-cell lymphoma (DLBCL), respectively.
Methods: Baseline 18F-FDG PET/CT (B-PET) and I-PET scans were performed in 112 patients with DLBCL. The prognostic value of I-PET using the Deauville five-point scale (D-5PS) criteria or percentage decrease in SUVmax (∆SUVmax) for GCB and non-GCB DLBCL were evaluated.
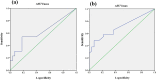
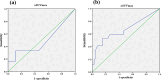
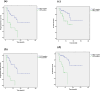
Results: A significant difference in progression-free survival (PFS) was found between GCB and non-GCB DLBCL patients (P < 0.05). Based on D-5PS criteria, I-PET was divided into positive (score > 3) and negative (score ≤ 3) subgroups. Results indicated that I-PET using D-5PS criteria was an independent predictor for PFS of GCB DLBCL (P < 0.05), but not for overall survival (OS) (P > 0.05). For non-GCB DLBCL, PFS and OS were significantly higher in I-PET negative group than I-PET positive group (P < 0.05). Receiver operating characteristic (ROC) curve analysis proved that I-PET using ΔSUVmax can also effectively predict PFS and OS of non-GCB DLBCL (P < 0.05), but not for GCB DLBCL (P > 0.05). Based on the optimal threshold found by ROC curve analysis, patients were dichotomized into ∆SUVmax high and low groups. Log-rank test and Cox regression demonstrated that the layered ∆SUVmax was predictive of PFS and OS in non-GCB DLBCL (P < 0.05).
Conclusions: I-PET may have different prognostic values for GCB and non-GCB DLBCL. Thus, the pathology type of DLBCL may be considered while using I-PET as a prognostic tool in the future.
Keywords: DLBCL; GCB; Non-GCB; PET/CT; R-CHOP.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The data collection and the methods used in this study were carried out in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The data collection program for this study was approved by Shanghai General Hospital Research Ethics Committee (No.2024064) with a waiver for individual informed consent for this retrospective study. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

