Integrating electrospun aligned fiber scaffolds with bovine serum albumin-basic fibroblast growth factor nanoparticles to promote tendon regeneration
- PMID: 39731092
- PMCID: PMC11673375
- DOI: 10.1186/s12951-024-03022-1
Integrating electrospun aligned fiber scaffolds with bovine serum albumin-basic fibroblast growth factor nanoparticles to promote tendon regeneration
Abstract
Background: Electrospun nanofiber scaffolds have been widely used in tissue engineering because they can mimic extracellular matrix-like structures and offer advantages including high porosity, large specific surface area, and customizable structure. In this study, we prepared scaffolds composed of aligned and random electrospun polycaprolactone (PCL) nanofibers capable of delivering basic fibroblast growth factor (bFGF) in a sustained manner for repairing damaged tendons.
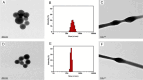
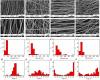
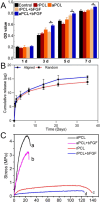
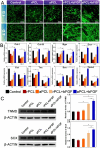
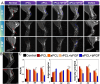
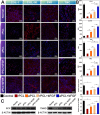
Results: Aligned and random PCL fiber scaffolds containing bFGF-loaded bovine serum albumin (BSA) nanoparticles (BSA-bFGF NPs, diameter 146 ± 32 nm) were fabricated, respectively. To validate the viability of bFGF-loaded aligned PCL nanofiber scaffold (aPCL + bFGF group) in tendon tissue engineering, we assessed the in vitro differentiation of human amniotic mesenchymal stem cells (hAMSCs) towards a tenogenic lineage and the in vivo regeneration of tendons using a rat Achilles tendon defect model. The encapsulated bFGF could be delivered in a sustained manner in vitro. The aPCL + bFGF scaffold promoted the in vitro differentiation of human amniotic mesenchymal stem cells (hAMSCs) towards a tenogenic lineage. In the repair of a rat Achilles tendon defect model, the aPCL + bFGF group showed a better repair effect. The scaffold offers a promising substrate for the regeneration of tendon tissue.
Conclusions: The aligned and random PCL fiber scaffolds containing bFGF nanoparticles were successfully prepared, and their physical and chemical properties were characterized. The aPCL + bFGF scaffold could promote the expression of the related genes and proteins of tendon-forming, facilitating tendon differentiation. In the rat Achilles tendon defect experiments, the aPCL + bFGF exhibited excellent tendon regeneration effects.
Keywords: Drug release; Electrospun nanofiber; Fiber orientation; Tendon repair; Tissue engineering.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This project was under the approval of the institutional review board at the Ethics Committee of the First Affiliated Hospital of Zunyi Medical University. Consent for publication: All participating patients signed the informed consents before enrolled in the trial. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
An epigenetic bioactive composite scaffold with well-aligned nanofibers for functional tendon tissue engineering.Acta Biomater. 2018 Jan 15;66:141-156. doi: 10.1016/j.actbio.2017.09.036. Epub 2017 Sep 28. Acta Biomater. 2018. PMID: 28963019
-
Living nanofiber yarn-based woven biotextiles for tendon tissue engineering using cell tri-culture and mechanical stimulation.Acta Biomater. 2017 Oct 15;62:102-115. doi: 10.1016/j.actbio.2017.08.043. Epub 2017 Aug 30. Acta Biomater. 2017. PMID: 28864251 Free PMC article.
-
Integration of mesenchymal stem cell sheet and bFGF-loaded fibrin gel in knitted PLGA scaffolds favorable for tendon repair.J Mater Chem B. 2019 Apr 7;7(13):2201-2211. doi: 10.1039/c8tb02759e. Epub 2019 Feb 27. J Mater Chem B. 2019. PMID: 32073579
-
Electrospun three-dimensional aligned nanofibrous scaffolds for tissue engineering.Mater Sci Eng C Mater Biol Appl. 2018 Nov 1;92:995-1005. doi: 10.1016/j.msec.2018.06.065. Epub 2018 Jun 30. Mater Sci Eng C Mater Biol Appl. 2018. PMID: 30184829 Review.
-
Advancements in biomaterials and scaffold design for tendon repair and regeneration.J Appl Biomater Funct Mater. 2025 Jan-Dec;23:22808000241310684. doi: 10.1177/22808000241310684. Epub 2025 May 26. J Appl Biomater Funct Mater. 2025. PMID: 40420476 Review.
References
-
- Sheean AJ, Arner JW, Bradley JP. Proximal hamstring tendon injuries: diagnosis and management. Arthroscopy. 2021;37(2):435–7. - PubMed
-
- Skinner S, Isaacs J. Extensor tendon injuries in the athlete. Clin Sports Med. 2020;39(2):259–77. - PubMed
-
- Kane SF, Olewinski LH, Tamminga KS. Management of chronic tendon injuries. Am Fam Physician. 2019;100(3):147–57. - PubMed
-
- Ruiz-Alonso S, Lafuente-Merchan M, Ciriza J, Saenz-Del-Burgo L, Pedraz JL. Tendon tissue engineering: cells, growth factors, scaffolds and production techniques. J Control Release. 2021;333:448–86. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

