Family concerns in organ donor conversations: a qualitative embedded multiple-case study
- PMID: 39731096
- PMCID: PMC11673370
- DOI: 10.1186/s13054-024-05198-2
Family concerns in organ donor conversations: a qualitative embedded multiple-case study
Abstract
Background: Listening and responding to family concerns in organ and tissue donation is generally considered important, but has never been researched in real time. We aimed to explore in real time, (a) which family concerns emerge in the donation process, (b) how these concerns manifest during and after the donor conversation, and (c) how clinicians respond to the concerns during the donor conversation.
Methods: A qualitative embedded multiple-case study in eight Dutch hospitals was conducted. Thematic analysis was performed based on audio recordings and direct observations of 29 donor conversations and interviews with the family members involved (n = 24).
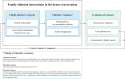
Results: Concerns clustered around six topics: 1) Life-event of a relative's death, 2) Dying well, 3) Tensions and fears about donation, 4) Experiences of time, 5) Procedural clarity, and 6) Involving (non-)present family. Most concerns occurred in topics 1 and 2. Clinicians mostly responded to concerns by providing information or immediate solutions, while sometimes acknowledgement sufficed. When concerns were highly charged with emotion, the clinicians' responses were less frequently attuned to families' needs. Cues of less clearly articulated concerns gained less follow-up. Then, concerns often remained or reappeared.
Conclusion: The identified concerns and the distinction between clearly and less clearly articulated concerns may prove valuable for clinicians to improve family support. We advise clinicians to engage with a curious, probing attitude to enhance the dialogue around concerns, elaborate on less clearly articulated concerns and identify the informational needs of the family.
Keywords: Clinical ethics; Communication; Intensive care unit; Organ donation; Qualitative research.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The Medical Review Ethics Committee East-Netherlands waived approval (2020–7044). The study also received approval from the local review committees of all participating hospitals. Written consent was obtained from all healthcare professionals and family members, except for three family members who provided recorded verbal consent. Informed consent was acquired from one representative on behalf of the whole family. Because some families were yet unaware of their relatives’ potential fatal diagnosis and possibility of donation, clinicians a priori informed them that the research focused on conversations within the ICU environment rather than specifically on donor conversations. Family’s verbal consent was obtained, followed several weeks later by full information, written or recorded verbal consent and the question to participate in an interview. Consent for publication: Not applicable. Conflict of interest: All authors have completed a disclosure form based on uniform ICMJE guidelines. As potential participants, donation intensivists did not have final decisional rights regarding the results and discussion sections of the manuscript. The authors have no competing interests to declare.
Figures
Similar articles
-
How clinicians discuss patients' donor registrations of consent and presumed consent in donor conversations in an opt-out system: a qualitative embedded multiple-case study.Crit Care. 2023 Jul 28;27(1):299. doi: 10.1186/s13054-023-04581-9. Crit Care. 2023. PMID: 37507800 Free PMC article.
-
Experiences of Families Following Organ Donation Consent: A Qualitative Systematic Review.Transplant Proc. 2021 Mar;53(2):501-512. doi: 10.1016/j.transproceed.2020.09.016. Epub 2021 Jan 19. Transplant Proc. 2021. PMID: 33483168
-
Requesting organ donation: an interview study of donor and nondonor families.Am J Crit Care. 1998 Jan;7(1):13-23. Am J Crit Care. 1998. PMID: 9429679
-
A Multicenter Qualitative Investigation of the Experiences and Perspectives of Substitute Decision Makers Who Underwent Organ Donation Decisions.Prog Transplant. 2018 Dec;28(4):343-348. doi: 10.1177/1526924818800046. Epub 2018 Sep 16. Prog Transplant. 2018. PMID: 30222045
-
"Effective" Requesting: A Scoping Review of the Literature on Asking Families to Consent to Organ and Tissue Donation.Transplantation. 2017 May;101(5S Suppl 1):S1-S16. doi: 10.1097/TP.0000000000001695. Transplantation. 2017. PMID: 28437367
References
-
- Kentish-Barnes N, Siminoff LA, Walker W, Urbanski M, Charpentier J, Thuong M, et al. A narrative review of family members’ experience of organ donation request after brain death in the critical care setting. Intensive Care Med. 2019;45(3):331–42. - PubMed
-
- Davidson JE, Aslakson RA, Long AC, Puntillo KA, Kross EK, Hart J, et al. Guidelines for family-centered care in the neonatal, pediatric, and adult ICU. Crit Care Med. 2017;45(1):103–28. - PubMed
-
- Curtis JR, Vincent J-L. Ethics and end-of-life care for adults in the intensive care unit. Lancet. 2010;376(9749):1347–53. - PubMed
-
- Werkgroep Ontwikkeling Kwaliteitsstandaard NTS. Kwaliteitsstandaard Donatie 2020 [Available from: https://www.transplantatiestichting.nl/files/2020-12/nts-kwaliteitsstand... (Accessed 20–4–2022).
MeSH terms
LinkOut - more resources
Full Text Sources
Medical