Enrolment patterns in a randomized controlled trial of probiotics in critically ill patients: a retrospective analysis of the PROSPECT trial
- PMID: 39731129
- PMCID: PMC11673600
- DOI: 10.1186/s13063-024-08701-w
Enrolment patterns in a randomized controlled trial of probiotics in critically ill patients: a retrospective analysis of the PROSPECT trial
Abstract
Background: Understanding site-related factors that influence enrolment within multicenter randomized controlled trials (RCT) may help reduce trial delays and cost over-runs and prevent early trial discontinuation. In this analysis of PROSPECT (Probiotics: Prevention of Severe Pneumonia and Endotracheal Colonization Trial), we describe patient enrolment patterns and examine factors influencing site-based monthly enrolment.
Design: Retrospective analysis of a multicenter RCT.
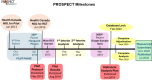
Methods: The PROSPECT multicenter RCT enrolled patients in the main trial from July 2015 to March 2019. We documented site characteristics and trial metrics including data from the methods center tracking documents, site-level data at trial initiation, screening logs submitted by research coordinators, and prospectively collected case report forms. In this retrospective analysis of trial data, we analyzed enrolment patterns across sites using negative binomial regression to explore the association between monthly enrolment rate accounting for number of ICU beds, site characteristics, and trial metrics.
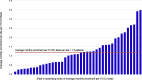
Results: Overall, 41 sites enrolling 2365 patients in the PROSPECT main trial were analyzed. After accounting for number of beds in each ICU, site launch early in the trial was associated with higher monthly enrolment rates, but time to first enrolment and research coordinator experience was not. We observed considerable variability in the number of active screening months and enrolment rates across sites.
Conclusion: These findings highlight the complexity of recruitment dynamics in critical care RCTs and emphasize the need for tailored approaches to trial planning and execution.
Trial registration: PROSPECT (Probiotics: Prevention of Severe Pneumonia and Endotracheal Colonization Trial): NCT02462590 (registered June 2, 2015).
Keywords: Critical illness; Enrolment; Randomized trial; Screening.
© 2024. Crown.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: We did not require ethical approval to carry out this pre-planned, post-hoc analysis since data included only site-level variables with no patient-level data or identifiers. Consent for publications: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

