Cancer Organoids as reliable disease models to drive clinical development of novel therapies
- PMID: 39731178
- PMCID: PMC11681695
- DOI: 10.1186/s13046-024-03258-7
Cancer Organoids as reliable disease models to drive clinical development of novel therapies
Abstract
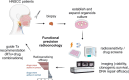
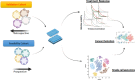
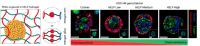
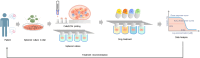
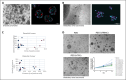
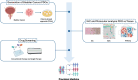
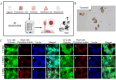
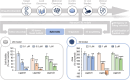
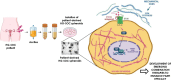
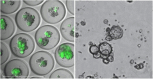
On September 23-24 (2024) the 6th Workshop IRE on Translational Oncology, titled "Cancer Organoids as Reliable Disease Models to Drive Clinical Development of Novel Therapies," took place at the IRCCS Regina Elena Cancer Institute in Rome. This prominent international conference focused on tumor organoids, bringing together leading experts from around the world.A central challenge in precision oncology is modeling the dynamic tumor ecosystem, which encompasses numerous elements that evolve spatially and temporally. Patient-derived 3D culture models, including organoids, explants, and engineered or bioprinted systems, have recently emerged as sophisticated tools capable of capturing the complexity and diversity of cancer cells interacting within their microenvironments. These models address critical unmet needs in precision medicine, particularly in aiding clinical decision-making. The rapid development of these human tissue avatars has enabled advanced modeling of cellular alterations in disease states and the screening of compounds to uncover novel therapeutic pathways.Throughout the event, distinguished speakers shared their expertise and research findings, illustrating how organoids are transforming our understanding of treatment resistance, metastatic dynamics, and the interaction between tumors and the surrounding microenvironment.This conference served as a pivotal opportunity to strengthen international collaborations and spark innovative translational approaches. Its goal was to accelerate the shift from preclinical research to clinical application, paving the way for increasingly personalized and effective cancer therapies.
Keywords: Cancer spheroid; Organoid; Patient-derived 3D culture model; Precision medicine; Preclinical models; Targeted therapy.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All the authors agree to publish this paper. Competing interests: The authors declare no competing interests.
Figures






References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

