Expression of claudin-18.2 in cholangiocarcinoma: a comprehensive immunohistochemical analysis from a German tertiary centre
- PMID: 39731204
- PMCID: PMC11791722
- DOI: 10.1111/his.15407
Expression of claudin-18.2 in cholangiocarcinoma: a comprehensive immunohistochemical analysis from a German tertiary centre
Abstract
Aims: Anti-claudin-18.2 (CLDN18.2) therapy was recently approved for the treatment of gastric or gastro-oesophageal junction adenocarcinoma. The aim of the present study was to investigate the expression of CLDN18.2 in cholangiocarcinoma (CCA) to determine whether there is a subgroup of patients who might also benefit from anti-CLDN18.2 therapy.
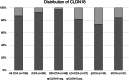
Methods and results: A tissue microarray (TMA) cohort of all CCA patients who underwent surgical resection with curative intent between August 2005 and December 2021 at University Hospital Frankfurt were immunohistochemically evaluated using the VENTANA® CLDN18 (43-14A) antibody. Tumour positivity for CLDN18.2 was determined as follows: ≥ 75% of tumour cells with moderate-to-strong CLDN18 membranous staining. In total, 160 patients with surgically resected CCA were suitable for immunohistochemistry (IHC) analysis. Of the patients, 13.1% (n = 21) showed moderate to strong membranous staining of VENTANA® CLDN18 antibody, while 86.9% (n = 139) were negative. Subtype analysis revealed strong differences in CLDN18 expression. Positive staining of CLDN18 could be observed in 26.5% (n = nine of 34) and 7.4% (n = seven of 95) of the perihilar (pCCA) and intrahepatic (iCCA) subgroup, respectively. CCA patients with CLDN18 expression had a more frequently intraoperative finding of distant metastasis (P = 0.002), lymph node metastasis (P = 0.008) and positive perineural invasion (Pn1) status (P = 0.022).
Conclusions: The present study suggests that a subset of patients with CCA exhibited a marked expression of CLDN18.2. These findings underline the need to perform a clinical study evaluating the efficacy of anti-CLDN18.2 therapy in patients suffering from CCA.
Keywords: CLDN18; biomarker; cholangiocarcinoma; surgical oncology; tight junctions.
© 2024 The Author(s). Histopathology published by John Wiley & Sons Ltd.
Conflict of interest statement
F.F. received travel support from Ipsen, Abbvie, Astrazeneca and speaker/advisory fees from AbbVie, MSD, Ipsen, Astrazeneca, Roche, BMS; S.Z. received consultancy and/or speaker's bureau, Abbvie, BioMarin, Boehringer Ingelheim, Gilead, GSK, Ipsen, Madrigal, MSD/Merck, NovoNordisk, SoBi and Theratechnologies; H.R., advisory board of Bristol‐Myers Squibb and Roche, received honoraria from Roche, Bristol‐Myers Squibb, Janssen‐Cilag, Novartis, Astra Zeneca, MCI, CHOP GmbH, Sanofi, Boehringer‐Ingelheim, GlaxoSmithKline, Merck and Diaceutics, received travel support from Philips, Roche and Bristol‐Myers Squibb and received grants from Bristol‐Myers Squibb; P.J.W. received consulting fees and honoraria for lectures by Bayer, Janssen‐Cilag, Novartis, Roche, MSD, Astellas Pharma, Bristol‐Myers Squibb, Thermo Fisher Scientific, Molecular Health, Guardant Health, Sophia Genetics, Qiagen, Eli Lilly, Myriad, Hedera Dx and Astra Zeneca; research support was provided by Astra Zeneca. The authors declare that there is no relationship relevant to the manuscripts’ subject. All other authors declare no conflicts of interest.
Figures

References
-
- Walter D, Ferstl P, Waidmann O et al. Cholangiocarcinoma in Germany: epidemiologic trends and impact of misclassification. Liver Int. 2019; 39; 316–323. - PubMed
-
- Kelley RK, Ueno M, Yoo C et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE‐966): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet (London, England). 2023; 401; 1853–1865. - PubMed
-
- Oh D‐Y, He AR, Qin S et al. A phase 3 randomized, double‐blind, placebo‐controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ‐1. J. Clin. Oncol. 2022; 40(4_suppl); 378.
-
- Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016; 17; 564–580. - PubMed
-
- Kyuno D, Takasawa A, Kikuchi S, Takemasa I, Osanai M, Kojima T. Role of tight junctions in the epithelial‐to‐mesenchymal transition of cancer cells. Biochim. Biophys. Acta Biomembr. 2021; 1863; 183503. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

