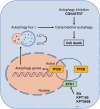
Autophagy modulates glioblastoma cell sensitivity to Selinexor-mediated XPO1 inhibition
- PMID: 39731209
- PMCID: PMC12187378
- DOI: 10.1093/neuonc/noae280
Autophagy modulates glioblastoma cell sensitivity to Selinexor-mediated XPO1 inhibition
Abstract
Background: Selinexor is a selective inhibitor of exportin-1 (XPO1), a key mediator of the nucleocytoplasmic transport for molecules critical to tumor cell survival. Selinexor's lethality is generally associated with the induction of apoptosis, and in some cases, with autophagy-induced apoptosis. We performed this study to determine Selinexor's action in glioblastoma (GBM) cells, which are notoriously resistant to apoptosis.
Methods: Patient-derived GBM cells were treated with Selinexor, and drug response and autophagy levels were monitored. Homozygous C528S XPO1 mutant GBM43 cells were generated by CRISPR/Cas9 editing. Single Selinexor or combination treatment with autophagy inhibitors was evaluated. In addition, bulk-tissue, single-cell, and spatial transcriptome were analyzed, and molecular docking was performed.
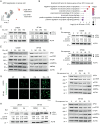
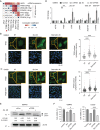
Results: Although all cell lines exhibited a dose- and time-dependent reduction of cell viability, the most profound molecular response to Selinexor was induction of autophagy instead of apoptosis. Selinexor-induced autophagy was an on-target consequence of XPO1 inhibition, and could be mitigated by expression of a mutant, Selinexor-resistant form of XPO1, and Selinexor-induced autophagy was related at least in part to nuclear trapping of the transcription factor TFEB. Furthermore, genetic or pharmacologic suppression of autophagy sensitized the cells to Selinexor-induced toxicity in association with the induction of apoptosis. Finally, in intracranial PDX studies, the combination of Selinexor with the autophagy inhibitor chloroquine significantly impeded tumor growth and extended mouse survival relative to single-agent treatment.
Conclusion: These results suggest that activation of autophagy confers a protective mechanism against Selinexor in GBM cells, and that the combination of Selinexor with autophagy inhibitors may serve as a viable means to enhance Selinexor-induced cell death.
Keywords: Selinexor; XPO1; autophagy; glioma; nuclear export.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures




References
-
- Ohno M, Fornerod M, Mattaj IW.. Nucleocytoplasmic transport: the last 200 nanometers. Cell. 1998;92(3):327–336. - PubMed
-
- Fukuda M, Asano S, Nakamura T, et al. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390(6657):308–311. - PubMed
-
- Wickramasinghe VO, Laskey RA.. Control of mammalian gene expression by selective mRNA export. Nat Rev Mol Cell Biol. 2015;16(7):431–442. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

