One-Pot Synthesis of Oxygen Vacancy-Rich Amorphous/Crystalline Heterophase CaWO4 Nanoparticles for Enhanced Radiodynamic-Immunotherapy
- PMID: 39731356
- PMCID: PMC11831444
- DOI: 10.1002/advs.202409551
One-Pot Synthesis of Oxygen Vacancy-Rich Amorphous/Crystalline Heterophase CaWO4 Nanoparticles for Enhanced Radiodynamic-Immunotherapy
Abstract
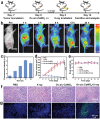
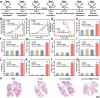
Radiodynamic therapy that employs X-rays to trigger localized reactive oxygen species (ROS) generation can tackle the tissue penetration issue of phototherapy. Although calcium tungstate (CaWO4) shows great potential as a radiodynamic agent benefiting from its strong X-ray absorption and the ability to generate electron-hole (e--h+) pairs, slow charge carrier transfer and fast e--h+ recombination greatly limit its ROS-generating performance. Herein, via a one-pot wet-chemical method, oxygen vacancy-rich amorphous/crystalline heterophase CaWO4 nanoparticles (Ov-a/c-CaWO4 NPs) with enhanced radiodynamic effect are synthesized for radiodynamic-immunotherapy of cancer. The phase composition and oxygen vacancy content of CaWO4 can be easily tuned by adjusting the solvothermal temperature. More intriguingly, the amorphous/crystalline interfaces and abundant oxygen vacancies accelerate charge carrier transfer and suppress e--h+ recombination, respectively, enabling synergistically improved ROS production from X-ray-irradiated Ov-a/c-CaWO4 NPs. In addition to directly inducing oxidative damage of cancer cells, radiodynamic generation of ROS also boosts immunogenic cell death to provoke a systemic antitumor immune response, thereby allowing the inhibition of both primary and distant tumors as well as cancer metastasis. This study establishes a synergistic enhancement strategy involving the integration of phase and defect engineering to improve the ROS generation capacity of radiodynamic-immunotherapeutic anticancer nanoagents.
Keywords: CaWO4 nanoparticles; enhanced radiodynamic effect; heterophase; oxygen vacancies; radiodynamic‐immunotherapy.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Ca2+- and cGAMP-Contained Semiconducting Polymer Nanomessengers for Radiodynamic-Activated Calcium Overload and Immunotherapy.Adv Sci (Weinh). 2025 Feb;12(6):e2411739. doi: 10.1002/advs.202411739. Epub 2024 Dec 16. Adv Sci (Weinh). 2025. PMID: 39679909 Free PMC article.
-
Low-dose X-ray radiodynamic therapy solely based on gold nanoclusters for efficient treatment of deep hypoxic solid tumors combined with enhanced antitumor immune response.Theranostics. 2023 Jan 22;13(3):1042-1058. doi: 10.7150/thno.78649. eCollection 2023. Theranostics. 2023. PMID: 36793856 Free PMC article.
-
Nanostructured CaWO4, CaWO4 : Pb2+ and CaWO4 : Tb3+ particles: polyol-mediated synthesis and luminescent properties.J Nanosci Nanotechnol. 2007 Feb;7(2):602-9. J Nanosci Nanotechnol. 2007. PMID: 17450802
-
Oxygen-Independent Radiodynamic Therapy: Radiation-Boosted Chemodynamics for Reprogramming the Tumor Immune Environment and Enhancing Antitumor Immune Response.ACS Appl Mater Interfaces. 2024 May 1;16(17):21546-21556. doi: 10.1021/acsami.4c00793. Epub 2024 Apr 16. ACS Appl Mater Interfaces. 2024. PMID: 38626342
-
Mitochondria-Targeted Nanosystem Enhances Radio-Radiodynamic-Chemodynamic Therapy on Triple Negative Breast Cancer.ACS Appl Mater Interfaces. 2023 May 10;15(18):21941-21952. doi: 10.1021/acsami.3c02361. Epub 2023 Apr 26. ACS Appl Mater Interfaces. 2023. PMID: 37099714
References
-
- a) Lin L., Wang S., Deng H., Yang W., Rao L., Tian R., Liu Y., Yu G., Zhou Z., Song J., Yang H.‐H., Chen Z.‐Y., Chen X., J. Am. Chem. Soc. 2020, 142, 15320; - PubMed
- b) Castano A. P., Mroz P., Hamblin M. R., Nat. Rev. Cancer 2006, 6, 535; - PMC - PubMed
- c) Tang Y., Bisoyi H. K., Chen X.‐M., Liu Z., Chen X., Zhang S., Li Q., Adv. Mater. 2023, 35, 2300232; - PubMed
- d) Wang J., Ding H., Zhu Y., Liu Y., Yu M., Cai H., Ao R., Huang H., Gong P., Liao Y., Chen Z., Lin L., Chen X., Yang H., Angew. Chem., Int. Ed. 2023, 135, e202302255; - PubMed
- e) Zhu Y., Gong P., Wang J., Cheng J., Wang W., Cai H., Ao R., Huang H., Yu M., Lin L., Chen X., Angew. Chem., Int. Ed. 2023, 62, e202218407. - PubMed
-
- a) Moore C. M., Pendse D., Emberton M., Nat. Clin. Pract. Urol. 2009, 6, 18; - PubMed
- b) Zeng S., Chen J., Gao R., Chen R., Xue Q., Ren Y., Liu L., Tang C., Hu H., Zeng N., Wen S., Zhang H., Liu C., Fang C., Adv. Mater. 2023, 36, 2308780; - PubMed
- c) Zhou H., Tang D., Yu Y., Zhang L., Wang B., Karges J., Xiao H., Nat. Commun. 2023, 14, 5350. - PMC - PubMed
- d) Li X., Lovell J. F., Yoon J., Chen X., Nat. Rev. Clin. Oncol. 2020, 17, 657; - PubMed
- e) Teng K.‐X., Niu L.‐Y., Yang Q.‐Z., J. Am. Chem. Soc. 2023, 145, 4081;
- f) Chen T., Hou P., Zhang Y., Ao R., Su L., Jiang Y., Zhang Y., Cai H., Wang J., Chen Q., Song J., Lin L., Yang H., Chen X., Angew. Chem., Int. Ed. 2021, 133, 15133. - PubMed
- g) Yang Y., Hu T., Bian Y., Meng F., Yu S., Li H., Zhang Q., Gu L., Weng X., Tan C., Liang R., Adv. Mater. 2023, 35, 2211205. - PubMed
-
- a) Fan W., Huang P., Chen X., Chem. Soc. Rev. 2016, 45, 6488; - PubMed
- b) Sorbellini E., Rucco M., Rinaldi F., Lasers Med. Sci. 2018, 33, 1431; - PMC - PubMed
- c) Yu M., Ye Z., Liu S., Zhu Y., Niu X., Wang J., Ao R., Huang H., Cai H., Liu Y., Chen X., Lin L., Angew. Chem., Int. Ed. 2024, 63, e202318155. - PubMed
-
- a) Begg A. C., Stewart F. A., Vens C., Nat. Rev. Cancer 2011, 11, 239; - PubMed
- b) Withers P. J., Bouman C., Carmignato S., Cnudde V., Grimaldi D., Hagen C. K., Maire E., Manley M., Du Plessis A., Stock S. R., Nat. Rev. Methods Primers 2021, 1, 18;
- c) Rabin O., Manuel Perez J., Grimm J., Wojtkiewicz G., Weissleder R., Nat. Mater. 2006, 5, 118; - PubMed
- d) Schaue D., McBride W. H., Nat. Rev. Clin. Oncol. 2015, 12, 527. - PMC - PubMed
- e) Baumann M., Krause M., Overgaard J., Debus J., Bentzen S. M., Daartz J., Richter C., Zips D., Bortfeld T., Nat. Rev. Cancer 2016, 16, 234. - PubMed
-
- a) Sun W., Shi T., Luo L., Chen X., Lv P., Lv Y., Zhuang Y., Zhu J., Liu G., Chen X., Adv. Mater. 2019, 31, 1808024; - PubMed
- b) Song L., Li P., Yang W., Lin X., Liang H., Chen X., Liu G., Li J., Yang H., Adv. Funct. Mater. 2018, 28, 1707496;
- c) Lu L., Sun M., Lu Q., Wu T., Huang B., Nano Energy 2021, 79, 105437.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
