Chemoenzymatic Formation of Oxa-Terpenoids by Sesqui- and Diterpene Synthase-Mediated Biotransformations with 9-Oxy-FPP Ether Derivatives
- PMID: 39731539
- PMCID: PMC11756643
- DOI: 10.1021/acs.biochem.4c00589
Chemoenzymatic Formation of Oxa-Terpenoids by Sesqui- and Diterpene Synthase-Mediated Biotransformations with 9-Oxy-FPP Ether Derivatives
Abstract
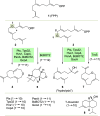
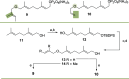
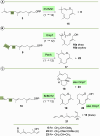
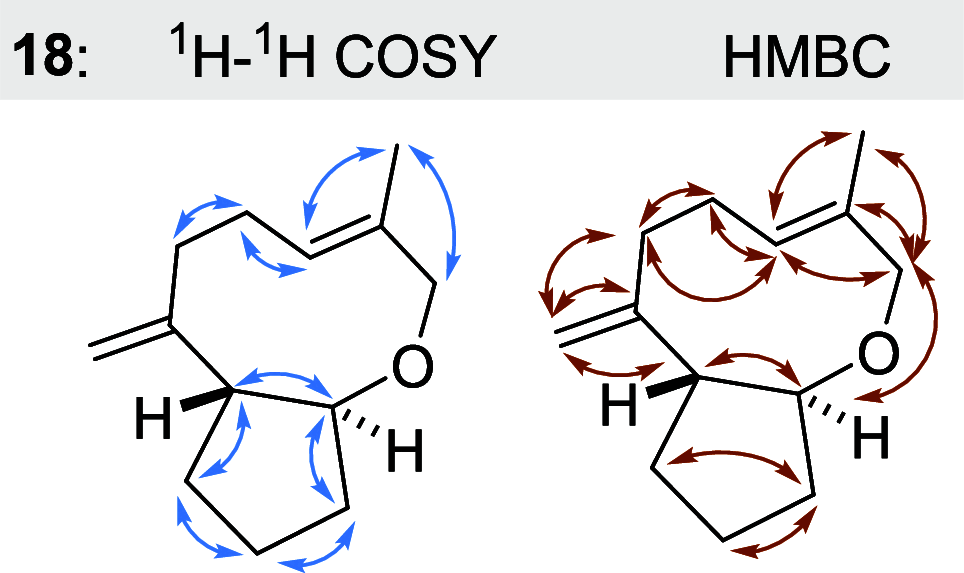
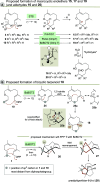
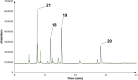
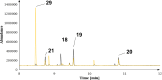
Farnesyl pyrophosphate derivatives bearing an additional oxygen atom at position 5 proved to be very suitable for expanding the substrate promiscuity of sesquiterpene synthases (STSs) and the formation of new oxygenated terpenoids. Insertion of an oxygen atom in position 9, however, caused larger restraints that led to restricted acceptance by STSs. In order to reduce some of the proposed restrictions, two FPP-ether derivatives with altered substitution pattern around the terminal olefinic double bond were designed. These showed improved promiscuity toward different STSs. Four new cyclized terpenoids with an embedded ether group were isolated and characterized. In the case of two cyclic enol ethers, also the corresponding "hydrolysis" products, linear hydroxyaldehydes, were isolated. Interestingly, all cyclization products originate from an initial 1 → 12 cyclization unprecedented when native farnesyl pyrophosphate serves as a substrate. We found that the most suitable FPP derivative with an additional oxygen at position 9 does not carry any methyl group on the terminal alkene, which likely reduces steric congestion when the preferred conformation for cyclization is adopted in the active site.
Copyright © 2024 The Authors. Published by American Chemical Society. This publication is licensed under CC-BY 4.0.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Cascón O.; Touchet S.; Miller D. J.; Gonzalez V.; Faraldos J. A.; Allemann R. K. Chem. Commun. 2012, 48, 9702–9704. 10.1039/c2cc35542f. - DOI - PubMed
- Touchet S.; Chamberlain K.; Woodcock C. M.; Miller D. J.; Birkett M. A.; Pickett J. A.; Allemann R. K. Chem. Commun. 2015, 51, 7550–7553. 10.1039/C5CC01814E. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

