A crucial active site network of titratable residues guides catalysis and NAD+ binding in human succinic semialdehyde dehydrogenase
- PMID: 39731543
- PMCID: PMC11681614
- DOI: 10.1002/pro.70024
A crucial active site network of titratable residues guides catalysis and NAD+ binding in human succinic semialdehyde dehydrogenase
Abstract
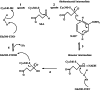
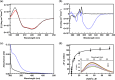
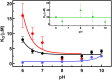
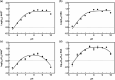
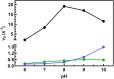
Human succinic semialdehyde dehydrogenase is a mitochondrial enzyme fundamental in the neurotransmitter γ-aminobutyric acid catabolism. It catalyzes the NAD+-dependent oxidative degradation of its derivative, succinic semialdehyde, to succinic acid. Mutations in its gene lead to an inherited neurometabolic rare disease, succinic semialdehyde dehydrogenase deficiency, characterized by mental and developmental delay. Due to the poor characterization of this enzyme, we carried out evolutionary and kinetic investigations to contribute to its functional behavior, a prerequisite to interpreting pathogenic variants. An in silico analysis shows that succinic semialdehyde dehydrogenases belong to two families, one human-like and the other of bacterial origin, differing in the oligomeric state and in a network of active site residues. This information is coupled to the biophysical-biochemical characterization of the human recombinant enzyme uncovering that (i) catalysis proceeds by an ordered bi-bi mechanism with NAD+ binding before the aldehyde that exerts a partial non-competitive inhibition; (ii) a stabilizing complex between the catalytic Cys340 and NAD+ is observed and interpreted as a protective mechanism; and (iii) a concerted non-covalent network assists the action of the catalytic residues Cys340 and Glu306. Through mutational analyses of Lys214, Glu306, Cys340, and Glu515 associated with pH studies, we showed that NAD+ binding is controlled by the dyad Lys214-Glu515. Moreover, catalysis is assured by proton transfer exerted by the same dyad networked with the catalytic Glu306, involved in catalytic Cys340 deprotonation/reprotonation. The identification of this weak bond network essential for cofactor binding and catalysis represents a first step to tackling the molecular basis for its deficiency.
Keywords: bioinformatic and evolutionary analysis; enzymatic mechanism; kinetics; succinic semialdehyde dehydrogenase; succinic semialdehyde dehydrogenase deficiency.
© 2024 The Author(s). Protein Science published by Wiley Periodicals LLC on behalf of The Protein Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
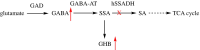


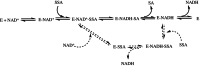

References
-
- Abraham MJ, Murtola T, Schulz R, Pall S, Smith JC, Hess B, et al. GROMACS: high perfomance molecular simulations through multi‐levels parallelism from laptops to supercomputers. SoftwareX. 2015;1–2:7–25.
-
- Ahn JW, Kim YG, Kim KJ. Crystal structure of non‐redox regulated ssadh from Escherichia coli . Biochem Biophys Res Commun. 2010;392(1):106–111. - PubMed
-
- Akaboshi S, Hogema BM, Novelletto A, Malaspina P, Salomons GS, Maropoulos GD, et al. Mutational spectrum of the succinate semialdehyde dehydrogenase (ALDH5A1) gene and functional analysis of 27 novel disease‐causing mutations in patients with SSADH deficiency. Hum Mutat. 2003;22(6):442–450. - PubMed
-
- Awoonor‐Williams E, Golosov AA, Hornak V. Benchmarking in silico tools for cysteine pK(a) prediction. J Chem Inf Model. 2023;63(7):2170–2180. - PubMed
-
- Baldy P. Metabolisme du γ‐aminobutyrate chez Agaricus bisporus III. La succinate‐semialdehyde:NAD(P)+ oxydoreductase. Physiol Planctarum. 1977;40(2):7.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

