Androgen receptor pathway inhibitors vs. docetaxel chemotherapy for metastatic hormone-sensitive and first-line castration resistant prostate cancer
- PMID: 39731623
- PMCID: PMC11682002
- DOI: 10.1007/s00345-024-05388-1
Androgen receptor pathway inhibitors vs. docetaxel chemotherapy for metastatic hormone-sensitive and first-line castration resistant prostate cancer
Abstract
Purpose: No currently available phase III trial compared docetaxel vs. androgen receptor pathway inhibitors (ARPI) regarding cancer-control outcomes in metastatic hormone-sensitive prostate cancer (mHSPC). Moreover, few is known about the effect of sequential therapies in mHSPC and subsequent metastatic castration resistant prostate cancer (mCRPC).
Methods: We relied on the FRAMCAP database and compared docetaxel vs. ARPI in mHSPC patients regarding time to mCRPC (ttCRPC) and overall survival (OS). Sensitivity analyses addressed high volume mHSPC patients. Finally, sequential therapies were compared regarding progression-free survival (PFS) and OS in first-line mCRPC.
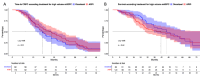
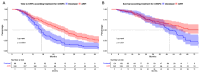
Results: Of 419 included mHSPC patients, 25% received docetaxel vs. 75% ARPI. ARPI patients were significantly older (71 vs. 66 years), and harbored lower baseline PSA (38 vs. 183 ng/ml, both p ≤ 0.002). Median ttCRPC was significantly longer for ARPI than for docetaxel-treated patients (30 vs. 17 months, hazard ratio [HR]: 0.49, p < 0.001). In OS analyses, ARPI patients also exhibited significantly longer OS, relative to docetaxel patients (96 vs. 50 months, HR: 0.67, p = 0.03). After multivariable adjustment in Cox regression models, no difference between both treatments remained in both analyses (all p > 0.05). In sensitivity analyses of high volume mHSPC patients only, also no ttCRPC or OS differences were observed for ARPI vs. docetaxel (all p > 0.05). Regarding sequential therapies, no PFS and OS differences were observed for all and specifically high volume mHSPC patients, when ARPI-ARPI vs. ARPI-docetaxel vs. docetaxel-ARPI treatments were compared (all p > 0.05).
Conclusion: In real-world setting, ARPI treatment performs comparable to docetaxel chemotherapy in mHSPC. Therefore, docetaxel should only be used in triplet therapy. Moreover, no differences for sequential therapies of ARPI/docetaxel combinations in first-line mCRPC were observed.
Keywords: ARPI; Apalutamide; Enzalutamide; mCRPC, high volume; mHSPC.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: Financial disclosures and Conflicts of interest: Mike Wenzel receives speaker honoraria or is consultant for Accord, Johnsen&Johnsen, Pfizer, MSD, Astra Zeneca, Ipsen, Benedikt Hoeh receives speaker honoraria or is consultant for Johnsen&Johnsen and Ipsen, Thomas Steuber receives speaker honoraria or is consultant for Amgen, Astellas, Astra Zeneca, Bayer, Johnson & Johnson, MSD, Novartis, Pfizer, Proteomedics, Sanofi, Markus Graefen receives speaker honoraria or is consultant for Intuitive Surgical, Metronic, Ipsen, Astellas, Johnson & Johnson und Takeda, Felix Chun receives speaker honoraria or is consultant for Astellas, AstraZeneca, Bayer, Johnson & Johnson, Lumenis, Molecular Health, Olympus, Pfizer, Philipp Mandel receives speaker honoraria or is consultant for AMGEN, Astellas, AstraZeneca, Bayer, IPSEN, Johnson & Johnson, MSD, Novartis, Orion, Pfizer. Conflicts of interest: Mike Wenzel receives speaker honoraria or is consultant for Accord, Johnsen&Johnsen, Pfizer, MSD, Astra Zeneca, Ipsen, Benedikt Hoeh receives speaker honoraria or is consultant for Johnsen&Johnsen and Ipsen, Thomas Steuber receives speaker honoraria or is consultant for Amgen, Astellas, Astra Zeneca, Bayer, Johnson & Johnson, MSD, Novartis, Pfizer, Proteomedics, Sanofi, Markus Graefen receives speaker honoraria or is consultant for Intuitive Surgical, Metronic, Ipsen, Astellas, Johnson & Johnson und Takeda, Felix Chun receives speaker honoraria or is consultant for Astellas, AstraZeneca, Bayer, Johnson & Johnson, Lumenis, Molecular Health, Olympus, Pfizer, Philipp Mandel receives speaker honoraria or is consultant for AMGEN, Astellas, AstraZeneca, Bayer, IPSEN, Johnson & Johnson, MSD, Novartis, Orion, Pfizer. Consent to participate: No informed consent was necessary according to the approval of the study by the ethic committee.
Figures
Similar articles
-
Treatment Patterns and Survival Among Veterans With De Novo Metastatic Hormone-Sensitive Prostate Cancer.JAMA Netw Open. 2025 May 1;8(5):e259433. doi: 10.1001/jamanetworkopen.2025.9433. JAMA Netw Open. 2025. PMID: 40338545 Free PMC article.
-
Lutetium-177 PSMA radioligand therapy in taxan-naive first- and second-line metastatic castration resistant prostate cancer after first-line ARPI therapy.Eur J Nucl Med Mol Imaging. 2025 May;52(6):2015-2022. doi: 10.1007/s00259-025-07076-7. Epub 2025 Jan 13. Eur J Nucl Med Mol Imaging. 2025. PMID: 39804375 Free PMC article.
-
Natural course of metastatic castration-resistant prostate cancer in the era of intensified androgen deprivation therapy in the hormone-sensitive setting.Prostate. 2024 Jun;84(9):888-892. doi: 10.1002/pros.24696. Epub 2024 Apr 1. Prostate. 2024. PMID: 38561317
-
Addition of New Androgen Receptor Pathway Inhibitors to Docetaxel and Androgen Deprivation Therapy in Metastatic Hormone-Sensitive Prostate Cancer: A Systematic Review and Metanalysis.Curr Oncol. 2022 Dec 4;29(12):9511-9524. doi: 10.3390/curroncol29120747. Curr Oncol. 2022. PMID: 36547161 Free PMC article.
-
Real-World Cabazitaxel Use and Outcomes in Metastatic Castrate-Resistant Prostate Cancer: The Impact of Response to First ARPI.Clin Genitourin Cancer. 2022 Oct;20(5):496.e1-496.e9. doi: 10.1016/j.clgc.2022.04.009. Epub 2022 Apr 26. Clin Genitourin Cancer. 2022. PMID: 35599196 Review.
Cited by
-
Treatment Patterns and Survival Among Veterans With De Novo Metastatic Hormone-Sensitive Prostate Cancer.JAMA Netw Open. 2025 May 1;8(5):e259433. doi: 10.1001/jamanetworkopen.2025.9433. JAMA Netw Open. 2025. PMID: 40338545 Free PMC article.
References
-
- Sharifi N, Gulley JL, Dahut WL (2005) Androgen deprivation therapy for prostate cancer. JAMA 294(2):238–244. 10.1001/jama.294.2.238 - PubMed
-
- Fizazi K, Tran N, Fein L et al (2017) Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 377(4):352–360. 10.1056/NEJMoa1704174 - PubMed
-
- Chi KN, Agarwal N, Bjartell A et al (2019) Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 381(1):13–24. 10.1056/NEJMoa1903307 - PubMed
-
- Armstrong AJ, Szmulewitz RZ, Petrylak DP et al (2019) ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol Off J Am Soc Clin Oncol 37(32):2974–2986. 10.1200/JCO.19.00799 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

