Comparative Effectiveness of Fluticasone Furoate/Umeclidinium/Vilanterol and Budesonide/Glycopyrrolate/Formoterol Fumarate among US Patients with Chronic Obstructive Pulmonary Disease
- PMID: 39731707
- PMCID: PMC11787206
- DOI: 10.1007/s12325-024-03088-1
Comparative Effectiveness of Fluticasone Furoate/Umeclidinium/Vilanterol and Budesonide/Glycopyrrolate/Formoterol Fumarate among US Patients with Chronic Obstructive Pulmonary Disease
Abstract
Introduction: Chronic obstructive pulmonary disease (COPD) is associated with exacerbations which can reduce quality of life and increase mortality. Single-inhaler triple therapy (SITT) is recommended for maintenance treatment of COPD among patients experiencing exacerbations despite dual-therapy use. This real-world comparative effectiveness study compared the impact of SITTs, fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI), and budesonide/glycopyrrolate/formoterol fumarate (BUD/GLY/FORM), on COPD exacerbations and mortality.
Methods: Medicare Fee-for-Service (FFS) patients with COPD initiated on FF/UMEC/VI or BUD/GLY/FORM were identified from the Komodo Research healthcare claims dataset (01/01/2016-12/31/2023). Overlap weighting based on high-dimensional propensity scores evaluated from patient characteristics was used to adjust for baseline confounding. Primary outcome was annualized rate of moderate-severe COPD exacerbations (per patient-year; PPY) compared using rate ratios (RRs) with 95% confidence intervals (CIs) from weighted Poisson regression models. Secondary and exploratory outcomes were risk of moderate-severe COPD exacerbations and all-cause mortality, respectively, evaluated using Kaplan-Meier analysis and hazard ratios (HR) with 95% CIs from Cox proportional hazard models. A secondary analysis was conducted among a mutually exclusive population with Medicare Advantage, Medicaid, or commercial insurance.
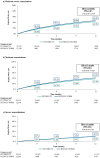
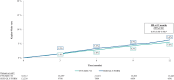
Results: Overall, 32,312 FF/UMEC/VI and 12,230 BUD/GLY/FORM Medicare FFS patients were included. After weighting, median follow-up was 9 months. Compared with BUD/GLY/FORM, FF/UMEC/VI users had a 12% lower rate of annualized moderate-severe COPD exacerbations [0.80 and 0.91 PPY; RR (95% CI): 0.88 (0.85-0.92); P < 0.001] and a 10% lower risk of moderate-severe exacerbations at 12 months post-initiation [HR (95% CI): 0.90 (0.87-0.93); P < 0.001], driven by moderate exacerbations. FF/UMEC/VI compared with BUD/GLY/FORM users had 11% lower risk of all-cause mortality at 12 months post-initiation [5.6% vs. 6.4%; HR (95% CI): 0.89 (0.80-0.98); P = 0.020]. Results were consistent among patients with Medicare Advantage, Medicaid, or commercial insurance.
Conclusions: In this real-world comparative effectiveness study, FF/UMEC/VI was associated with significantly lower rate and risk of COPD exacerbations than BUD/GLY/FORM.
Keywords: Budesonide/glycopyrrolate/formoterol fumarate; Chronic obstructive pulmonary disease; Exacerbations; Fluticasone furoate/umeclidinium/vilanterol; Real-world comparative effectiveness study.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: David Mannino is a consultant for AstraZeneca, the COPD Foundation, Genentech, GSK, Regeneron, and Up-to-Date. David Mannino is also an expert witness on behalf of people suing the tobacco and vaping industries. Stephen Weng, Sergio Forero-Schwanhaeuser, Chris H Compton, Stephen G Noorduyn, and Rosirene Paczkowski are employees of GSK and/or hold financial equities in GSK. Stephen G Noorduyn is also a PhD candidate at McMaster University. Guillaume Germain, Julien Boudreau, Anabelle Tardif-Samson, François Laliberté, and Patrick Gravelle are employees of Groupe d’analyse, which received funding from GSK to conduct this study. Ethical Approval: This study complied with all applicable laws regarding patient privacy, as described in the Declaration of Helsinki. No direct patient contact or primary collection of individual human patient data has occurred in this study. This study used existing, fully de-identified data that complied with the requirements of the Health Insurance Portability and Accountability Act and the patient(s) cannot be identified, directly or through identifiers. Study results were in tabular form and aggregate analyses that omit patient identification; therefore, informed consent, ethics committee or Institutional Review Board approval were not required.
Figures

References
-
- Lange P, Ahmed E, Lahmar ZM, Martinez FJ, Bourdin A. Natural history and mechanisms of COPD. Respirology. 2021;26(4):298–321. 10.1111/resp.14007. - PubMed
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2024. https://goldcopd.org/2024-gold-report/. Accessed April 29, 2024.
-
- Centers for Disease Control and Prevention. National Center for Health Statistics. National Health Interview Survey, 2019–2022. Analysis by the American Lung Assocation Research and Program Services Division using SPSS software. https://www.lung.org/research/trends-in-lung-disease/copd-trends-brief/d.... Accessed Sept 23, 2024.
-
- Gillen EM, Mercado N, Sunkari K. Medicare enrollees with COPD compared to the general population. 2021. https://avalere.com/insights/medicare-enrollees-with-copd-compared-to-th.... Accessed Sept 23, 2024.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

