Shared genetic architecture and bidirectional clinical risks within the psycho-metabolic nexus
- PMID: 39731856
- PMCID: PMC11743124
- DOI: 10.1016/j.ebiom.2024.105530
Shared genetic architecture and bidirectional clinical risks within the psycho-metabolic nexus
Abstract
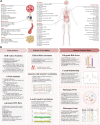
Background: Increasing evidence suggests a complex interplay between psychiatric disorders and metabolic dysregulations. However, most research has been limited to specific disorder pairs, leaving a significant gap in our understanding of the broader psycho-metabolic nexus.
Methods: This study leveraged large-scale cohort data and genome-wide association study (GWAS) summary statistics, covering 8 common psychiatric disorders and 43 metabolic traits. We introduced a comprehensive analytical strategy to identify shared genetic bases sequentially, from key genetic correlation regions to local pleiotropy and pleiotropic genes. Finally, we developed polygenic risk score (PRS) models to translate these findings into clinical applications.
Findings: We identified significant bidirectional clinical risks between psychiatric disorders and metabolic dysregulations among 310,848 participants from the UK Biobank. Genetic correlation analysis confirmed 104 robust trait pairs, revealing 1088 key genomic regions, including critical hotspots such as chr3: 47588462-50387742. Cross-trait meta-analysis uncovered 388 pleiotropic single nucleotide variants (SNVs) and 126 shared causal variants. Among variants, 45 novel SNVs were associated with psychiatric disorders and 75 novel SNVs were associated with metabolic traits, shedding light on new targets to unravel the mechanism of comorbidity. Notably, RBM6, a gene involved in alternative splicing and cellular stress response regulation, emerged as a key pleiotropic gene. When psychiatric and metabolic genetic information were integrated, PRS models demonstrated enhanced predictive power.
Interpretation: The study highlights the intertwined genetic and clinical relationships between psychiatric disorders and metabolic dysregulations, emphasising the need for integrated approaches in diagnosis and treatment.
Funding: The National Key Research and Development Program of China (2023YFC2506200, SHH). The National Natural Science Foundation of China (82273741, SY).
Keywords: Clinical risks; Genetic association; Metabolism; Polygenic risk score; Psychiatric disorder.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests No conflicts of interest, financial or otherwise, are declared by the authors. All authors were not paid to write this article by a pharmaceutical company or other agency.
Figures




References
-
- Goldfarb M., De Hert M., Detraux J., et al. Severe mental illness and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2022;80:918–933. - PubMed
-
- Chen W., Feng J., Jiang S., et al. Mendelian randomization analyses identify bidirectional causal relationships of obesity with psychiatric disorders. J Affect Disord. 2023;339:807–814. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

