Longitudinal synaptic loss versus tau Braak staging in amnestic mild cognitive impairment
- PMID: 39732507
- PMCID: PMC11848342
- DOI: 10.1002/alz.14412
Longitudinal synaptic loss versus tau Braak staging in amnestic mild cognitive impairment
Abstract
Introduction: The longitudinal progression of synaptic loss in Alzheimer's disease (AD) and how it is affected by tau pathology remains poorly understood.
Methods: Thirty patients with amnestic mild cognitive impairment (aMCI) and 26 healthy controls underwent cognitive evaluations and tau, synaptic vesicle protein 2A (SV2A), and amyloid positron emission tomography. Twenty-one aMCI underwent 2-year follow-up (FU) investigations.
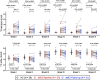
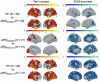
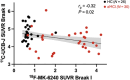
Results: Tau levels in aMCI increased longitudinally in Braak regions III through VI but not in Braak regions I and II. SV2A decreased longitudinally in all Braak regions in aMCI. Baseline tau was negatively associated with longitudinal SV2A loss in early Braak regions and with SV2A at FU across regions. Baseline tau and longitudinal change in SV2A were associated with longitudinal cognitive decline.
Discussion: Tau accumulation reaches a plateau in early Braak regions already in the aMCI stage of AD. In early Braak regions, the association between baseline tau and longitudinal SV2A loss might reflect synaptic dysfunction caused by tau pathology.
Highlights: Tau accumulation reached a plateau in early Braak regions in amnestic mild cognitive impairment (aMCI) patients. aMCI patients show widespread longitudinal decrease in synaptic vesicle protein 2A (SV2A) over 2 years. Baseline tau was predictive for longitudinal SV2A loss. The tau-SV2A relation showed individual variability and was negative across patients. Baseline tau and longitudinal SV2A change were associated with change in cognition.
Keywords: Alzheimer's disease; cognition; positron emission tomography; synaptic density; synaptic vesicle protein 2A; tau.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
K.V.L. performed this study as senior investigator of FWO Flanders. K.V.L. is an advisory board member of Cerveau‐Lantheus and has received fees through KU Leuven for consultancy activities for GE Healthcare. K.V.L. and M.K. have performed contract research through KU Leuven for Merck, Janssen Pharmaceuticals, UCB, Syndesi, Eikonizo, GE Healthcare, Cerevel, BMS, and Curasen. No other potential conflicts of interest relevant to this article exist. Author disclosures are available in the supporting information.
Figures


Similar articles
-
Fibre density and cross-section associate with hallmark pathology in early Alzheimer's disease.Alzheimers Res Ther. 2025 Apr 5;17(1):73. doi: 10.1186/s13195-025-01710-0. Alzheimers Res Ther. 2025. PMID: 40188035 Free PMC article.
-
Assessing Synaptic Density in Alzheimer Disease With Synaptic Vesicle Glycoprotein 2A Positron Emission Tomographic Imaging.JAMA Neurol. 2018 Oct 1;75(10):1215-1224. doi: 10.1001/jamaneurol.2018.1836. JAMA Neurol. 2018. PMID: 30014145 Free PMC article.
-
In vivo measurement of widespread synaptic loss in Alzheimer's disease with SV2A PET.Alzheimers Dement. 2020 Jul;16(7):974-982. doi: 10.1002/alz.12097. Epub 2020 May 13. Alzheimers Dement. 2020. PMID: 32400950 Free PMC article.
-
In vivo imaging of synaptic density in neurodegenerative disorders with positron emission tomography: A systematic review.Ageing Res Rev. 2024 Feb;94:102197. doi: 10.1016/j.arr.2024.102197. Epub 2024 Jan 22. Ageing Res Rev. 2024. PMID: 38266660
-
Clinical Correlates of the PET-based Braak Staging Framework in Alzheimer's Disease.J Prev Alzheimers Dis. 2024;11(2):414-421. doi: 10.14283/jpad.2024.15. J Prev Alzheimers Dis. 2024. PMID: 38374747 Review.
Cited by
-
Fibre density and cross-section associate with hallmark pathology in early Alzheimer's disease.Alzheimers Res Ther. 2025 Apr 5;17(1):73. doi: 10.1186/s13195-025-01710-0. Alzheimers Res Ther. 2025. PMID: 40188035 Free PMC article.
-
Synaptic loss pattern is constrained by brain connectome and modulated by phosphorylated tau in Alzheimer's disease.Nat Commun. 2025 Jul 10;16(1):6356. doi: 10.1038/s41467-025-61497-4. Nat Commun. 2025. PMID: 40640137 Free PMC article.
-
Prediction of longitudinal synaptic loss in Alzheimer's disease using tau PET and plasma biomarkers.Alzheimers Dement. 2025 May;21(5):e70333. doi: 10.1002/alz.70333. Alzheimers Dement. 2025. PMID: 40432308 Free PMC article.
-
Synapse vulnerability and resilience across the clinical spectrum of dementias.Nat Rev Neurol. 2025 Jul;21(7):353-369. doi: 10.1038/s41582-025-01094-7. Epub 2025 May 22. Nat Rev Neurol. 2025. PMID: 40404832 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

