Onco-mNGS facilitates rapid and precise identification of the etiology of fever of unknown origin: a single-centre prospective study in North China
- PMID: 39732661
- PMCID: PMC11682622
- DOI: 10.1186/s12879-024-10383-3
Onco-mNGS facilitates rapid and precise identification of the etiology of fever of unknown origin: a single-centre prospective study in North China
Abstract
Objectives: Delayed diagnosis of patients with Fever of Unknown Origin has long been a daunting clinical challenge. Onco-mNGS, which can accurately diagnose infectious agents and identify suspected tumor signatures by analyzing host chromosome copy number changes, has been widely used to assist identifying complex etiologies. However, the application of Onco-mNGS to improve FUO etiological screening has never been studied before.
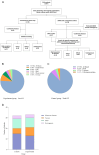
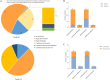
Methods: In this single-centre prospective study, we included 65 patients with classic FUO, who were randomly divided into control group (sample cultivation) and mNGS group (cultivation + Onco-mNGS). We analyzed the infectious agents and symbiotic microbiological, tumor and clinical data of both groups.
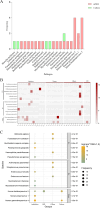
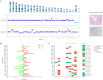
Results: Infection-related pathogenic detection efficiency rose from 25% (control group) to 48.48% (experimental group). Seven patients with chromosome copy number changes had later been confirmed tumors, indicating a 100% of clinical concordance rate of Onco-mNGS for tumors. In addition, the time frame for diagnosing or ruling out infection/tumor with Onco-mNGS had greatly reduced to approximately 2 days, which was 7.34 days earlier than that in the control group.
Conclusions: Onco-mNGS is an ideal rapid diagnostic aid to assist improving the early diagnostic efficiency of FUO-associated diseases.
Keywords: Diagnostic criteria; Etiology; Fever of unknown origin; Onco-mNGS.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The studies involving human participants were reviewed and approved by the board of Ethics at The First Hospital of China Medical University. The patients provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. The study was conducted in accordance with the International Conference on Harmonization, Good Clinical Practice Guidelines, and the Declaration of Helsinki. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources

