Association between antinuclear antibodies status and preterm birth in Japanese pregnant women: a prospective cohort study from Adjunct Study of the Japan Environment and Children's Study
- PMID: 39732712
- PMCID: PMC11682645
- DOI: 10.1186/s12884-024-07084-9
Association between antinuclear antibodies status and preterm birth in Japanese pregnant women: a prospective cohort study from Adjunct Study of the Japan Environment and Children's Study
Abstract
Background: Antinuclear antibodies (ANA) are important biomarkers for the diagnosis of autoimmune diseases; however, the general population also tests positive at a low frequency, especially in women. Although the effects of various autoimmune diseases on pregnancy outcomes have been studied, the association of ANA with pregnancy outcomes in healthy individuals is unclear. Preterm birth (PTB), a major cause of neonatal death or long-term health problems, is a complex condition with a multifactorial etiology, and the underlying mechanism remains unclear. The present Adjunct Study aimed to determine the association between ANA and PTB in pregnant Japanese women based on a data analysis of the Japan Environment and Children's Study.
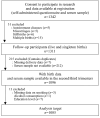
Methods: In a prospective cohort design, we analyzed the demographic and pregnancy outcome data of 1085 pregnant Japanese women who were recruited between January 2011 and March 2014 in the Kumamoto University target area. Demographic data were collected using self-administered questionnaires and physician records. A serum ANA titer of ≥ 1:40 was defined as positive. Statistical analysis was performed by logistic regression analysis with PTB as the objective variable.
Results: The PTB rate was significantly higher in those who were ANA-positive (adjusted odds ratio, 2.06; 95% confidence interval, 1.09-3.87) than in those who were not.
Conclusions: This study suggests that ANA positivity in the first trimester of pregnancy is associated with an increased risk of PTB.
Keywords: Antinuclear antibodies; Japan; Pregnancy outcome; Pregnant women; Preterm Birth; Prospective studies.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Ethics Review Committee for Epidemiological Research of the Ministry of the Environment (approval number: 100910001) and the Ethics Review Committee of the affiliated institutions (approval number: 590). Written informed consent was obtained from all participants. This study was conducted in accordance with the Declaration of Helsinki and other national regulations and guidelines. To ensure protection of privacy rights, the data were de-linked from personal identifiers and strictly handled. Consent for publication: This study is not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Kavanaugh A, Tomar R, Reveille J, Solomon DH, Homburger HA. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. American college of pathologists. Arch Pathol Lab Med. 2000;124:71–81. - PubMed
-
- Conrad N, Misra S, Verbakel JY, Verbeke G, Molenberghs G, Taylor PN, et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet. 2023;401:1878–90. - PubMed
-
- Sakkas LI, Chikanza IC. Sex bias in immune response: it is time to include the sex variable in studies of autoimmune rheumatic diseases. Rheumatol Int. 2023. 10.1007/s00296-023-05446-8. - PubMed
-
- Bundhun PK, Soogund MZ, Huang F. Impact of systemic lupus erythematosus on maternal and fetal outcomes following pregnancy: a meta-analysis of studies published between years 2001–2016. J Autoimmun. 2017;79:17–27. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources