A new evidence-based design-of-experiments approach for optimizing drug delivery systems with exemplification by emulsion-derived Vancomycin-loaded PLGA capsules
- PMID: 39732761
- PMCID: PMC11682087
- DOI: 10.1038/s41598-024-82496-3
A new evidence-based design-of-experiments approach for optimizing drug delivery systems with exemplification by emulsion-derived Vancomycin-loaded PLGA capsules
Abstract
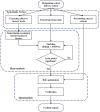
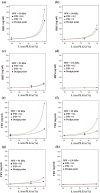
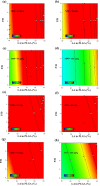
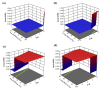
This paper introduces an evidence-based, design-of-experiments (DoE) approach to analyze and optimize drug delivery systems, ensuring that release aligns with the therapeutic window of the medication. First, the effective factors and release data of the system are extracted from the literature and meta-analytically undergo regression modeling. Then, the interaction and correlation of the factors to each other and the release amount are quantitatively assessed. Finally, the factors are numerically and graphically optimized via linking the meta-analyzed release data and the well-documented therapeutic window of the drug, followed by verification. For a more in-depth explanation, the introduced approach is exemplified by a drug delivery, consisting of emulsion-derived poly lactic-co-glycolic acid-vancomycin (PLGA-VAN) capsules for treating Staphylococcus Aureus-induced osteomyelitis. Novel and validated findings for the model system, along with the thorough architecture of the introduced approach, suggest its potential applicability for any delivery systems with sufficient reliable data in the literature.
Keywords: Burst and sustained release kinetics; Local controlled drug delivery systems; Pharmacokinetics.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

