Inhibiting CFTR through inh-172 in primary neutrophils reveals CFTR-specific functional defects
- PMID: 39732786
- PMCID: PMC11682091
- DOI: 10.1038/s41598-024-82535-z
Inhibiting CFTR through inh-172 in primary neutrophils reveals CFTR-specific functional defects
Abstract
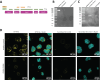
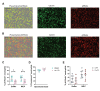
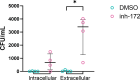
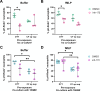
The lungs of people with cystic fibrosis (PwCF) are characterized by recurrent bacterial infections and inflammation. Infections in cystic fibrosis (CF) are left unresolved despite excessive neutrophil infiltration. The role of CFTR in neutrophils is not fully understood. In this study, we aimed to assess which antimicrobial functions are directly impaired by loss of CFTR function in neutrophils. In order to do so, we used a specific inhibitor of CFTR ion channel activity, inh-172. CF neutrophils from PwCF harboring severe CFTR mutations were additionally isolated to further discern CFTR-specific functional defects. We evaluated phagocytosis, reactive oxygen species (ROS) production, neutrophil elastase (NE) and myeloperoxidase (MPO) exocytosis and bacterial killing. The inh-172 model identified decreased acidification of the phagosome, increased bacterial survival and decreased ROS production upon stimulation. In PwCF neutrophils, we observed reduced degranulation of both NE and MPO. When co-culturing neutrophils with CF sputum supernatant and airway epithelial cells, the extent of phagocytosis was reduced, underscoring the importance of recreating an inflammatory environment as seen in PwCF lungs to model immune responses in vitro. Despite low CFTR expression in blood neutrophils, functional defects were found in inh-172-treated and CF neutrophils. The inh-172 model disregards donor variability and allows pinpointing neutrophil functions directly impaired by dysfunctional CFTR.
Keywords: CFTR; Cystic fibrosis; Inh-172; Neutrophils; Phagocytosis.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: M.S.C. and M. Boon report speaker fees from Vertex Pharmaceuticals. M. Boon is member of the ERN-LUNG (European Reference Network on rare respiratory diseases) network. All other authors declare that they do not have any competing interests. Ethical approval: The study was conducted according to the guidelines of the Declaration of Helsinki, and the protocol (S57236 [ML11095]) was approved by the ethical committee of KU Leuven/UZ Leuven. Informed consent was obtained from all subjects in this study.
Figures



References
-
- Cystic Fibrosis Mutations Database. http://www.genet.sickkids.on.ca/ (2011).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

