New class of thio/semicarbazide-based benzyloxy derivatives as selective class of monoamine oxidase-B inhibitors
- PMID: 39732801
- PMCID: PMC11682371
- DOI: 10.1038/s41598-024-82771-3
New class of thio/semicarbazide-based benzyloxy derivatives as selective class of monoamine oxidase-B inhibitors
Abstract
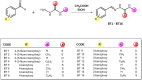
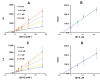
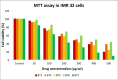
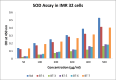
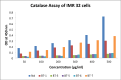
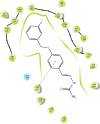
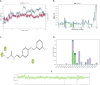
Sixteen thio/semicarbazide-based benzyloxy derivatives (BT1-BT16) were synthesized and evaluated for their inhibitory activities against monoamine oxidases (MAOs). Most compounds showed better inhibitory activity against MAO-B than against MAO-A. BT1, BT3, and BT5 showed the greatest inhibitory activity with an identical IC50 value of 0.11 µM against MAO-B, followed by BT6 and BT7 (IC50 = 0.12 µM) and BT2 (IC50 = 1.68 µM). The selectivity index of BT5 was the highest (363.64) for MAO-B, whereas that of BT1 was 88.73. BT1 and BT5 were reversible MAO-B inhibitors, based on the results of dialysis experiments. In inhibition kinetics, BT1 and BT5 were competitive MAO-B inhibitors with Ki values of 0.074 ± 0.0020 and 0.072 ± 0.0079 µM, respectively. Additionally, in the in-vitro parallel artificial membrane penetration assay, BT1 and BT5 crossed the blood-brain barrier. Cytotoxicity and possible neuroprotective effects of the lead compounds were assessed using IMR 32 cells. Levels of the antioxidant superoxide dismutase, catalase, and glutathione peroxidase in IMR 32 cells were increased by pretreatment with lead compounds. Five lead molecules (BT1, BT3, BT5, BT6, and BT7) were used for the docking studies. A significant pi-pi interaction with Tyr 326 was observed and molecular dynamics studies were performed for the most promising BT1-MAO-B complex. These results suggested that BT1 and BT5 could be used therapeutically for the treatment of various neurodegenerative diseases.
Keywords: Benzyloxy benzene-based thio/Semicarbazide derivatives; Kinetics; Molecular dynamics; Monoamine oxidase; Reversibility.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures





References
-
- Rodríguez-Enríquez, F. et al. Novel coumarin-pyridazine hybrids as selective MAO-B inhibitors for the Parkinson’s disease therapy. Bioorg. Chem.104, 104203 (2020). - PubMed
-
- Abid, S. M. A. et al. Sulfonyl hydrazones derived from 3-formylchromone as non-selective inhibitors of MAO-A and MAO-B: synthesis, molecular modelling and in-silico ADME evaluation. Bioorg. Chem.75, 291–302 (2017). - PubMed
-
- Chen, J. J. & Swope, D. M. Clinical pharmacology of rasagiline: a novel, second-generation propargylamine for the treatment of Parkinson disease. J. Clin. Pharmacol.45, 878–894 (2005). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

