iPSC-derived human sensory neurons reveal a subset of TRPV1 antagonists as anti-pruritic compounds
- PMID: 39732807
- PMCID: PMC11682095
- DOI: 10.1038/s41598-024-82549-7
iPSC-derived human sensory neurons reveal a subset of TRPV1 antagonists as anti-pruritic compounds
Abstract
Signaling interplay between the histamine 1 receptor (H1R) and transient receptor potential cation channel subfamily V member 1 (TRPV1) in mediating histaminergic itch has been well-established in mammalian models, but whether this is conserved in humans remains to be confirmed due to the difficulties in obtaining human sensory neurons (SNs) for experimentation. Additionally, previously reported species-specific differences in TRPV1 function indicate that use of human SNs is vital for drug candidate screening to have a higher chance of identifying clinically effective TRPV1 antagonists. In this study, we built a histamine-dependent itch model using peripheral SNs derived from human induced pluripotent stem cells (hiPSC-SNs), which provides an accessible source of human SNs for pre-clinical drug screening. We validated channel functionality using immunostaining, calcium imaging, and multielectrode array (MEA) recordings, and confirmed the interdependence of H1R and TRPV1 signalling in human SNs. We further tested the amenability of our model for pre-clinical studies by screening multiple TRPV1 antagonists in parallel, identifying SB366791 as a potent inhibitor of H1R activation and potential candidate for alleviating histaminergic itch. Notably, some of the results using our model corroborated with efficacy and side effect findings from human clinical trials, underscoring the importance of this species-specific platform. Taken together, our results present a robust in vitro human model for histaminergic itch, which can be used to further interrogate the molecular basis of human SN function as well as screen for TRPV1 activity-modifying compounds for a number of clinical indications.
Keywords: Histamine receptor; Human pluripotent stem cells; Itch; MEA; Sensory neurons; TRPV1; TRPV1 antagonist.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
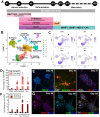


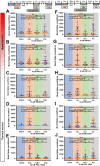
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

