Intervention effect of regulating GABA-A receptor activity on the formation of experimental abdominal aortic aneurysm in rats
- PMID: 39732918
- PMCID: PMC11682254
- DOI: 10.1038/s41598-024-82913-7
Intervention effect of regulating GABA-A receptor activity on the formation of experimental abdominal aortic aneurysm in rats
Abstract
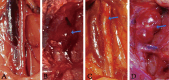
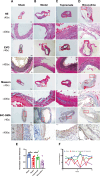
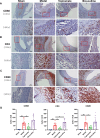
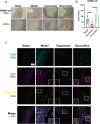
Abdominal aortic aneurysm is a potentially fatal vascular inflammatory disease characterized by infiltration of various inflammatory cells.The GABA-A receptor is expressed in many inflammatory cells such as macrophages and T cells and has anti-inflammatory and antioxidant effects. Therefore, the GABA-A receptor may become a potential therapeutic target for abdominal aortic aneurysms. The purpose of this study was to investigate the effect of regulating the activity of the GABA-A receptor on the formation of experimental abdominal aortic aneurysm in rats. In this study, the abdominal aortic aneurysm model of rats was established by aorta intracavitary perfusion of elastase combined with aorta extracavitary infiltration of calcium chloride. GABA-A receptor agonist (topiramate) and antagonist (bicuculline) were used to treating the abdominal aortic aneurysm model rats, which were divided into sham operation group, model group, topiramate group, and bicuculline group(n = 10). Histopathology, immunohistochemistry, fluorescence quantitative PCR, Western blotting, ELISA and Gelatine zymogram were used to study. Regulation of GABA-A receptor activity can interfere with the development and severity of abdominal aortic aneurysms in rats. The GABA-A receptor agonist topiramate reduces the infiltration of inflammatory cells, particularly T cells, into the abdominal aortic wall, while also modulating the balance of Th1/Th2 cytokines in peripheral blood, leading to a significant reduction in inflammatory responses. Additionally, topiramate decreases the secretion of matrix metalloproteinases MMP2 and MMP9, thereby inhibiting extracellular matrix degradation and slowing the progression of aneurysms. In contrast, the GABA-A receptor antagonist bicuculline exacerbates inflammation and promotes aneurysm development. At the molecular level, the mechanisms of action of the GABA-A receptor agonist topiramate and the antagonist bicuculline may involve inhibition or activation of the p38 MAPK signaling pathway. Regulation of GABA-A receptor activity can effectively intervene in the occurrence and development of abdominal aortic aneurysms in rats.
Keywords: Abdominal aortic aneurysm; Bicuculline; GABA; GABA-A receptor; Topiramate.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All experiments were performed according to guidelines established by the Institutional Animal Care and Use Ethical Committee of North Sichuan Medical College (Sichuan, China). Consent for publication: The submission to this journal has been read and approved by all coauthors.
Figures
Similar articles
-
Establishment of a New Abdominal Aortic Aneurysm Model in Rats by a Retroperitoneal Approach.Front Cardiovasc Med. 2022 Feb 23;9:808732. doi: 10.3389/fcvm.2022.808732. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35282381 Free PMC article.
-
RAGE deficiency ameliorates abdominal aortic aneurysm progression.Inflamm Res. 2025 Apr 17;74(1):63. doi: 10.1007/s00011-025-02027-2. Inflamm Res. 2025. PMID: 40244438
-
Levo-Tetrahydropalmatine Attenuates Progression of Abdominal Aortic Aneurysm in an Elastase Perfusion Rat Model via Suppression of Matrix Metalloproteinase and Monocyte Chemotactic Protein-1.Med Sci Monit. 2018 Feb 1;24:652-660. doi: 10.12659/msm.906153. Med Sci Monit. 2018. Retraction in: Med Sci Monit. 2022 Nov 09;28:e938902. doi: 10.12659/MSM.938902. PMID: 29388563 Free PMC article. Retracted.
-
AGE-RAGE stress play a role in aortic aneurysm: A comprehensive review and novel potential therapeutic target.Rev Cardiovasc Med. 2019 Dec 30;20(4):201-208. doi: 10.31083/j.rcm.2019.04.57. Rev Cardiovasc Med. 2019. PMID: 31912711 Review.
-
Advantages of an antagonist: bicuculline and other GABA antagonists.Br J Pharmacol. 2013 May;169(2):328-36. doi: 10.1111/bph.12127. Br J Pharmacol. 2013. PMID: 23425285 Free PMC article. Review.
References
-
- De Freitas, S., D’Ambrosio, N. & Fatima, J. Infrarenal Abdominal aortic Aneurysm[J]. Surg. Clin. North. Am.103 (4), 595–614 (2023). - PubMed
-
- Jeanmonod, D., Yelamanchili, V-S. & Jeanmonod, R. Abdominal Aortic Aneurysm Rupture[J]. (2023). - PubMed
-
- Liu, X. et al. Platelet protects angiotensin II-driven abdominal aortic aneurysm formation through inhibition of inflammation[J]. Exp. Gerontol., 159111703. (2022). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

