Exploring potential multiple molecular biomarkers that predict treatment response in patients with lupus nephritis
- PMID: 39733104
- PMCID: PMC11682382
- DOI: 10.1038/s41598-024-83057-4
Exploring potential multiple molecular biomarkers that predict treatment response in patients with lupus nephritis
Abstract
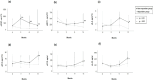
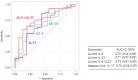
Limited knowledge exists regarding biomarkers that predict treatment response in Lupus nephritis (LN). We aimed to identify potential molecular biomarkers to predict treatment response in patients with LN. We enrolled 66 patients with active LN who underwent renal biopsy upon enrollment. Serum and urine samples were collected longitudinally, and we measured 12 biomarkers in each sample using a multiplex immunofluorescence assay. These biomarkers included monocyte chemoattractant protein-1 (MCP-1), interferon gamma-induced protein 10 (IP-10), interferon-γ (IFN-γ), interleukin 6 (IL-6), interleukin 16 (IL-16), interleukin 17 (IL-17), interleukin 23 (IL-23), tumor necrosis factor receptor II (TNF-RII), vascular cell adhesion molecule 1 (VCAM-1), retinol-binding protein 4 (RBP 4), vitamin D binding protein (VDBP), and neutrophil gelatinase-associated lipocalin (NGAL). Patients were categorized into two groups based on their 1-year treatment response to Mycophenolate mofetil (MMF)-based therapy: 50 responders and 16 non-responders. Only urine IL-17 (uIL-17) showed baseline level differences between the two groups, with higher in responders. In ROC curve analyses assessing the predictive performance of biomarkers, baseline uIL-17 and changes in uIL-6 and uIL-23 levels at 3 months could predict the 1-year treatment response, showing AUC values of 0.70 (95% CI 0.54-0.87), 0.70 (0.54-0.86), and 0.71 (0.57-0.85), respectively. Combining uIL-6 and uIL-23 into a model improved predictability, achieving an AUC of 0.75 (0.61-0.90). Baseline uIL-17 levels and early changes in uIL-6 and uIL-23 could serve as potential biomarkers to predict 1-year treatment response in lupus nephritis patients receiving MMF-based therapy.
Keywords: Biomarker; Lupus nephritis; Treatment response.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: Human samples were collected in accordance with approved guidelines set by the human ethics committee (Hanyang University Medical Center, Approval Number: HYUH2017-08-035). Informed consent was obtained from all participants before participation. This study adhered to the ethical principles outlined in the Declaration of Helsinki. Consent to participate: Written informed consent was obtained from all participants.
Figures
References
-
- Bastian, H. M. et al. Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus11, 152–160 (2002). - PubMed
-
- Kalloo, S., Aggarwal, N., Mohan, P. & Radhakrishnan, J. Lupus nephritis: treatment of resistant disease. Clin. J. Am. Soc. Nephrol.8, 154–161. 10.2215/CJN.05870612 (2013). - PubMed
-
- Liu, Z. et al. Multitarget therapy for induction treatment of lupus nephritis: a randomized trial. Ann. Intern. Med.162, 18–26. 10.7326/M14-1030 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

