Enhancing antibody levels and T cell activity of quadrivalent influenza vaccine by combining it with CpG HP021
- PMID: 39733119
- PMCID: PMC11682164
- DOI: 10.1038/s41598-024-83026-x
Enhancing antibody levels and T cell activity of quadrivalent influenza vaccine by combining it with CpG HP021
Abstract
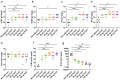
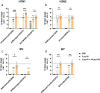
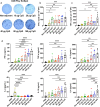
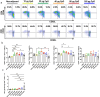
Influenza virus infections are a serious danger to people's health worldwide as they are responsible for seasonal flu outbreaks. There is an urgent need to improve the effectiveness and durability longevity of the immune response to influenza vaccines. We synthesized the CpG HP021 and examined the impact of it on the immune response to an influenza vaccine. In BALB/c mice, hemagglutination inhibition (HI) titers to the vaccine were increased four- to eightfold against H1N1, H3N2, BV, and BY viruses by 3 μg IIV4 + 40 μg CpG HP021 compared with those of the non-adjuvanted IIV4 group, and the CpG HP021 group had a broader HI activity. Additionally, the immune response was directed towards Type 1 T helper (Th1) cells due to the CpG HP021 adjuvant. The CpG HP021-adjuvanted IIV4 induced a higher number of T cells secreting interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α), and increased the percentage of effector memory T cells in mice. In SD rats, the immune responses induced by IIV4 with CpG HP021 were similar to those in BALB/c mice. The development of CpG HP021 may expand the options for adjuvants in vaccines against infectious diseases.
Keywords: Antibody; CpG adjuvant; Hemagglutination inhibition; Quadrivalent influenza vaccine; T cell immunity.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: CpG HP021 is patented by Jiangsu Taipurui Biotechnology Co., Ltd. All other authors declare no competing interests.
Figures

Similar articles
-
Effects of different adjuvants in the context of intramuscular and intranasal routes on humoral and cellular immune responses induced by detergent-split A/H3N2 influenza vaccines in mice.Clin Vaccine Immunol. 2012 Feb;19(2):209-18. doi: 10.1128/CVI.05441-11. Epub 2011 Dec 21. Clin Vaccine Immunol. 2012. PMID: 22190392 Free PMC article.
-
Combination adjuvants for the induction of potent, long-lasting antibody and T-cell responses to influenza vaccine in mice.Vaccine. 2008 Jan 24;26(4):552-61. doi: 10.1016/j.vaccine.2007.11.054. Epub 2007 Dec 7. Vaccine. 2008. PMID: 18162266
-
Broadly Protective CD8+ T Cell Immunity to Highly Conserved Epitopes Elicited by Heat Shock Protein gp96-Adjuvanted Influenza Monovalent Split Vaccine.J Virol. 2021 May 24;95(12):e00507-21. doi: 10.1128/JVI.00507-21. Print 2021 May 24. J Virol. 2021. PMID: 33827939 Free PMC article.
-
Boosting the immune response in COVID-19 vaccines via an Alum:CpG complex adjuvant.Antiviral Res. 2024 Sep;229:105954. doi: 10.1016/j.antiviral.2024.105954. Epub 2024 Jul 2. Antiviral Res. 2024. PMID: 38964615
-
Mastoparan-7 adjuvanted COBRA H1 and H3 hemagglutinin influenza vaccines.Sci Rep. 2024 Jun 14;14(1):13800. doi: 10.1038/s41598-024-64351-7. Sci Rep. 2024. PMID: 38877101 Free PMC article.
Cited by
-
One Health adjuvant selection for vaccines against zoonotic infections.Explor Med. 2025;6:1001316. doi: 10.37349/emed.2025.1001316. Epub 2025 May 7. Explor Med. 2025. PMID: 40444176 Free PMC article.
References
-
- Belongia, E. A. et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis.16, 942–951. 10.1016/s1473-3099(16)00129-8 (2016). - PubMed
-
- Peng, S. et al. Particulate Alum via Pickering Emulsion for an Enhanced COVID-19 Vaccine Adjuvant. Adv. Mater.32, e2004210. 10.1002/adma.202004210 (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

