Histological effects of combined therapy involving scar resection, decellularized scaffolds, and human iPSC-NS/PCs transplantation in chronic complete spinal cord injury
- PMID: 39733145
- PMCID: PMC11682313
- DOI: 10.1038/s41598-024-82959-7
Histological effects of combined therapy involving scar resection, decellularized scaffolds, and human iPSC-NS/PCs transplantation in chronic complete spinal cord injury
Abstract
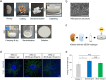
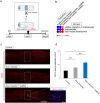
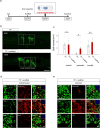
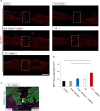
Chronic complete spinal cord injury (SCI) is difficult to treat because of scar formation and cavitary lesions. While human iPS cell-derived neural stem/progenitor cell (hNS/PC) therapy shows promise, its efficacy is limited without the structural support needed to address cavitary lesions. Our study investigated a combined approach involving surgical scar resection, decellularized extracellular matrix (dECM) hydrogel as a scaffold, and hNS/PC transplantation. To mitigate risks such as prion disease associated with spinal cord-derived dECM, we used kidney-derived dECM hydrogel. This material was chosen for its biocompatibility and angiogenic potential. In vitro studies with dorsal root ganglia (DRG) confirmed its ability to support axonal growth. In a chronic SCI rat model, scar resection enhanced the local microenvironment by increasing neuroprotective microglia and macrophages, while reducing inhibitory factors that prevent axonal regeneration. The combination of scar resection and dECM hydrogel further promoted vascular endothelial cell migration. These changes improved the survival of transplanted hNS/PCs and facilitated host axon regeneration. Overall, the integrated approach of scar resection, dECM hydrogel scaffolding, and hNS/PC transplantation has been proven to be a more effective treatment strategy for chronic SCI. However, despite histological improvements, no functional recovery occurred and further research is needed to enhance functional outcomes.
Keywords: Cell transplantation; Chronic phase; Scaffold; Scar resection; Spinal cord injury.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
-
- Nagoshi, N. et al. Phase I/II study of intrathecal administration of recombinant human hepatocyte growth factor in patients with acute spinal cord injury: A double-blind, randomized clinical trial of safety and efficacy. J. Neurotrauma37, 1752–1758 (2020). - PubMed
-
- Honmou, O. et al. Intravenous infusion of auto serum-expanded autologous mesenchymal stem cells in spinal cord injury patients: 13 case series. Clin. Neurol. Neurosurg.203, 106565 (2021). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- JP24ym0126118/Japan Agency for Medical Research and Development
- 24bm1223008/Japan Agency for Medical Research and Development
- JP24ym0126118/Japan Agency for Medical Research and Development
- 23hk0102089h0001/Japan Agency for Medical Research and Development
- 23hk0102089h0001/Japan Agency for Medical Research and Development
- 23hk0102089h0001/Japan Agency for Medical Research and Development
- JP24ym0126118/Japan Agency for Medical Research and Development
- 24bm1223008/Japan Agency for Medical Research and Development
- JP24ym0126118/Japan Agency for Medical Research and Development
- JP24ym0126118/Japan Agency for Medical Research and Development
- KAKENHI grant number 23K24464/Japan Society for the Promotion of Science
- KAKENHI grant number 23K24464/Japan Society for the Promotion of Science
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

