Harnessing Raman spectroscopy and multimodal imaging of cartilage for osteoarthritis diagnosis
- PMID: 39733214
- PMCID: PMC11682361
- DOI: 10.1038/s41598-024-83155-3
Harnessing Raman spectroscopy and multimodal imaging of cartilage for osteoarthritis diagnosis
Abstract
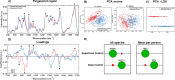
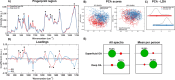
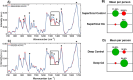
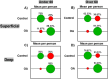
Osteoarthritis (OA) is a complex disease of cartilage characterised by joint pain, functional limitation, and reduced quality of life with affected joint movement leading to pain and limited mobility. Current methods to diagnose OA are predominantly limited to X-ray, MRI and invasive joint fluid analysis, all of which lack chemical or molecular specificity and are limited to detection of the disease at later stages. A rapid minimally invasive and non-destructive approach to disease diagnosis is a critical unmet need. Label-free techniques such as Raman Spectroscopy (RS), Coherent anti-Stokes Raman scattering (CARS), Second Harmonic Generation (SHG) and Two Photon Fluorescence (TPF) are increasingly being used to characterise cartilage tissue. However, current studies are based on whole tissue analysis and do not consider the different and structurally distinct layers in cartilage. In this work, we use Raman spectroscopy to obtain signatures from the superficial (top) and deep (bottom) layer of healthy and osteoarthritic cartilage samples from 64 patients (19 control and 45 OA). Spectra were acquired both in the 'fingerprint' region from 700 to 1720 cm- 1 and high-frequency stretching region from 2500 to 3300 cm- 1. Principal component and linear discriminant analysis was used to identify the peaks that contributed significantly to classification accuracy of the different samples. The most pronounced differences were observed at the proline (855 cm- 1 and 921 cm- 1) and hydroxyproline (877 cm- 1 and 938 cm- 1), sulphated glycosaminoglycan (sGAG) (1064 cm- 1 and 1380 cm- 1) frequencies for both control and OA as well as the 1245 cm- 1 and 1272 cm- 1, 1320 cm- 1 and 1345 cm- 1, 1451 cm- 1 collagen modes were altered in OA samples, consistent with expected collagen structural changes. Classification accuracy based on Raman fingerprint spectral analysis of superficial and deep layer cartilage for controls was found to be 97% and 93% on using individual/all spectra and, 100% and 95% on using mean spectra per patient, respectively. OA diseased cartilage was classified with an accuracy of 88% and 84% for individual/all spectra, and 96% and 95% for mean spectra per patient based on analysis of the superficial and the deep layers, respectively. Raman spectra from the C-H stretching region (2500-3300 cm- 1) resulted in high classification accuracy for identification of different layers and OA diseased cartilage but low accuracy for controls. Differential changes in superficial and deep layer cartilage signatures were observed with age (under 60 and over 60 years), in contrast, less significant differences were observed with gender. Prominent chemical changes in the different layers of cartilage were preliminarily imaged using CARS, SHG and TPF. Cell clustering was observed in OA together with differences in pericellular matrix and collagen structure in the superficial and the deep layers correlating with the Raman spectral analysis. The current study demonstrates the potential of Raman Spectroscopy and multimodal imaging to interrogate cartilage tissue and provides insight into the chemical and structural composition of its different layers with significant implications for OA diagnosis for an increasing aging demographic.
Keywords: Deep tissue imaging; Fingerprint Raman; Multimodal imaging; cartilage layers; osteoarthritis diagnosis.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
-
- Hunter, D. J., March, L. & Chew, M. Osteoarthritis in 2020 and beyond: a Lancet Commission. Lancet396, 1711–1712. 10.1016/S0140-6736(20)32230-3 (2020). - PubMed
-
- Wittenauer, R., Smith, L. & Aden K. M.
-
- Mandelbaum, B. & Waddell, D. Etiology and pathophysiology of osteoarthritis. Orthopedics28, s207–214. 10.3928/0147-7447-20050202-05 (2005). - PubMed
-
- Bachrach, N. M. et al. Changes in proteoglycan synthesis of chondrocytes in articular cartilage are associated with the time-dependent changes in their mechanical environment. J. Biomech.28, 1561–1569. 10.1016/0021-9290(95)00103-4 (1995). - PubMed
-
- Mow, V. C., Ratcliffe, A. & Poole, A. R. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials13, 67–97. 10.1016/0142-9612(92)90001-5 (1992). - PubMed
Publication types
MeSH terms
Grants and funding
- EP/T020997/1/Engineering and Physical Sciences Research Council
- EP/T020997/1/Engineering and Physical Sciences Research Council
- EP/T020997/1/Engineering and Physical Sciences Research Council
- EP/T020997/1/Engineering and Physical Sciences Research Council
- EP/T020997/1/Engineering and Physical Sciences Research Council
LinkOut - more resources
Full Text Sources
Medical

