ASB1 engages with ELOB to facilitate SQOR ubiquitination and H2S homeostasis during spermiogenesis
- PMID: 39733518
- PMCID: PMC11743861
- DOI: 10.1016/j.redox.2024.103484
ASB1 engages with ELOB to facilitate SQOR ubiquitination and H2S homeostasis during spermiogenesis
Abstract
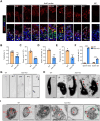
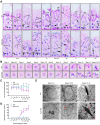
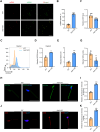
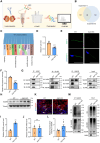
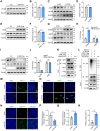
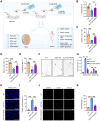
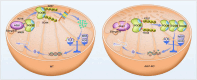
Male infertility, frequently driven by oxidative stress, impacts half of infertile couples globally. Despite its significance, the precise mechanisms governing this process remain elusive. In this study, we demonstrate that ASB1, the substrate recognition subunit of a ubiquitin ligase, is highly expressed in the mouse testis. Mice lacking the Asb1 gene exhibit severe fertility impairment, characterized by oligoasthenoteratozoospermia. Subsequent investigations unveiled that Asb1 knockout (Asb1-KO) mice encountered excessive oxidative stress and decreased hydrogen sulfide (H2S) levels in their testes, and severe sperm DNA damage. Notably, the compromised fertility and sperm quality in Asb1-KO mice was significantly ameliorated by administering NaHS, a H2S donor. Mechanistically, ASB1 interacts with ELOB to induce the instability of sulfide-quinone oxidoreductase (SQOR) by enhancing its K48-linked ubiquitination on residues K207 and K344, consequently triggering proteasomal degradation. This process is crucial for preserving H2S homeostasis and redox balance. Overall, our findings offer valuable insights into the role of ASB1 during spermiogenesis and propose H2S supplementation as a promising therapeutic approach for oxidative stress-related male infertility.
Keywords: ASB1; Hydrogen sulfide; Oxidative stress; Polyubiquitination; Spermiogenesis.
Copyright © 2024 Nanjing Medical University. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no competing interests.
Figures


Similar articles
-
Testis-expressed protein 33 is not essential for spermiogenesis and fertility in mice.Mol Med Rep. 2021 May;23(5):317. doi: 10.3892/mmr.2021.11956. Epub 2021 Mar 24. Mol Med Rep. 2021. PMID: 33760102 Free PMC article.
-
Proteolytic degradation of heat shock protein A2 occurs in response to oxidative stress in male germ cells of the mouse.Mol Hum Reprod. 2017 Feb 10;23(2):91-105. doi: 10.1093/molehr/gaw074. Mol Hum Reprod. 2017. PMID: 27932549
-
Hydrogen Sulfide As a Potential Target in Preventing Spermatogenic Failure and Testicular Dysfunction.Antioxid Redox Signal. 2018 Jun 1;28(16):1447-1462. doi: 10.1089/ars.2016.6968. Epub 2017 Oct 16. Antioxid Redox Signal. 2018. PMID: 28537489
-
[The Roles of N6-Methyladenosine Modification and Its Regulators in Male Reproduction].Sichuan Da Xue Xue Bao Yi Xue Ban. 2024 May 20;55(3):527-534. doi: 10.12182/20240560103. Sichuan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38948273 Free PMC article. Review. Chinese.
-
Hydrogen sulfide and its potential as a possible therapeutic agent in male reproduction.Front Endocrinol (Lausanne). 2024 Sep 11;15:1427069. doi: 10.3389/fendo.2024.1427069. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39324123 Free PMC article. Review.
Cited by
-
Dcun1d3 is dispensable for spermatogenesis and male fertility in mice.Am J Clin Exp Immunol. 2025 Jun 15;14(3):127-137. doi: 10.62347/BZPE6333. eCollection 2025. Am J Clin Exp Immunol. 2025. PMID: 40689315 Free PMC article.
-
CRL2LRRC41-Mediated DDX5 Ubiquitination Enhances Interaction with ELAVL1 Preventing NOG mRNA Degradation and Sustaining Proliferation and Migration of Human Spermatogonial Stem Cell-Like Cell Line.BMC Biol. 2025 Aug 7;23(1):247. doi: 10.1186/s12915-025-02363-z. BMC Biol. 2025. PMID: 40775760 Free PMC article.
-
Development and validation a nomogram to predict long-term mortality risks of PRISm and mild-to-moderate COPD based on NHANES 2007-2012.Sci Rep. 2025 May 8;15(1):16000. doi: 10.1038/s41598-025-94399-y. Sci Rep. 2025. PMID: 40341137 Free PMC article.
-
Testis-enriched Spsb1 is not required for spermatogenesis and fertility in mice.Am J Transl Res. 2025 Mar 15;17(3):1824-1833. doi: 10.62347/JFJX7128. eCollection 2025. Am J Transl Res. 2025. PMID: 40226024 Free PMC article.
-
ITGA5 promotes cervical cancer progression by regulating IMP3 recruitment of HK2 mRNA.Am J Transl Res. 2025 Apr 15;17(4):3031-3049. doi: 10.62347/GGVE9638. eCollection 2025. Am J Transl Res. 2025. PMID: 40385029 Free PMC article.
References
-
- Agarwal A., Baskaran S., Parekh N., Cho C.L., Henkel R., Vij S., Arafa M., Panner Selvam M.K., Shah R. Male infertility. Lancet. 2021;397:319–333. - PubMed
-
- Dai C., Zhang Z., Shan G., Chu L.T., Huang Z., Moskovtsev S., Librach C., Jarvi K., Sun Y. Advances in sperm analysis: techniques, discoveries and applications. Nat. Rev. Urol. 2021;18:447–467. - PubMed
-
- Li L., Yuan Y., Sha J. Potential clinical value of in vitro spermatogenesisdagger. Biol. Reprod. 2022;107:95–100. - PubMed
-
- Bisht S., Faiq M., Tolahunase M., Dada R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017;14:470–485. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

