Live Akkermansia muciniphila boosts dendritic cell retinoic acid synthesis to modulate IL-22 activity and mitigate colitis in mice
- PMID: 39734222
- PMCID: PMC11684322
- DOI: 10.1186/s40168-024-01995-7
Live Akkermansia muciniphila boosts dendritic cell retinoic acid synthesis to modulate IL-22 activity and mitigate colitis in mice
Erratum in
-
Correction: Live Akkermansia muciniphila boosts dendritic cell retinoic acid synthesis to modulate IL-22 activity and mitigate colitis in mice.Microbiome. 2025 Feb 19;13(1):54. doi: 10.1186/s40168-025-02060-7. Microbiome. 2025. PMID: 39972522 Free PMC article. No abstract available.
Abstract
Background: The interplay between gut microbiota and immune responses is crucial in ulcerative colitis (UC). Though Akkermansia muciniphila (Akk) shows therapeutic potential, the mechanisms remain unclear. This study sought to investigate differences in therapeutic efficacy among different forms or strains of Akk and elucidate the underlying mechanisms.
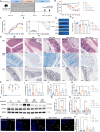
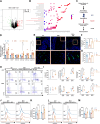
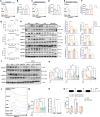
Results: Employing a dextran sulfate sodium (DSS)-induced colitis mouse model, we assessed Akk's impact on colitis using cellular cytokine analysis, immune phenotyping, proteomics, and biochemical methods. Our results suggest that treatment with live Akk effectively reduced colitis in the DSS-induced model, whereas heat-inactivated Akk did not yield the same results. Notably, Akk exhibited protective properties by promoting the secretion of IL-22 by Group 3 innate lymphoid cells (ILC3s), as evidenced by the absence of protection in IL-22 knockout mice. Additionally, Akk augmented the population of CD103+CD11b- dendritic cells (DCs) and enhanced their retinoic acid (RA) synthesis through the modulation of RALDH2, a crucial enzyme in RA metabolism. The depletion of RALDH2 in DCs diminished Akk's protective properties and impaired IL-22-mediated mucosal healing. Mechanistically, Akk activated RA production in DCs by enhancing the JAK2-STAT3 signaling pathway. Additionally, various strains of Akk may exhibit differing abilities to alleviate colitis, with the novel strain Am06 derived from breast milk showing consistent efficacy similar to the reference strain.
Conclusions: In summary, our findings indicate that certain strains of Akk may mitigate colitis through the promotion of RA synthesis and IL-22 secretion, underscoring the potential efficacy of Akk as a therapeutic intervention for the management of UC. Video Abstract.
Keywords: Akkermansia muciniphila; Dendritic cells; Group 3 innate lymphoid cells; IL-22; Retinoic acid; STAT3; Ulcerative colitis.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments conducted adhered strictly to the Guidelines for Care and Use of Laboratory Animals as prescribed by the National Institutes of Health. Every animal study protocol utilized in this research was granted approval by the Institutional Animal Care and Use Committee at Southern Medical University (Approval No. K2019090). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Le Berre C, Honap S, Peyrin-Biroulet L. Ulcerative colitis. The Lancet. 2023;402:571–84. - PubMed
-
- Glymenaki M, Singh G, Brass A, Warhurst G, McBain AJ, Else KJ, et al. Compositional changes in the gut mucus microbiota precede the onset of colitis-induced inflammation. Inflamm Bowel Dis. 2017;23:912–22. - PubMed
-
- van de Guchte M, Mondot S, Doré J. Dynamic properties of the intestinal ecosystem call for combination therapies, targeting inflammation and microbiota, in ulcerative colitis. Gastroenterology. 2021;161:1969-1981.e12. - PubMed
-
- Gilliland A, Chan JJ, De Wolfe TJ, Yang H, Vallance BA. Pathobionts in Inflammatory Bowel Disease: Origins, Underlying Mechanisms, and Implications for Clinical Care. Gastroenterology. 2024;166:44–58. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
- 2022M721510/China Postdoctoral Science Foundation
- 82373282/National Natural Science Foundation of China
- 81970465/National Natural Science Foundation of China
- 2022A1515012649/Science and Technology Planning Project of Guangdong Province
- 2017B020209003/Science and Technology Planning Project of Guangdong Province
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

