Targeting AURKA with multifunctional nanoparticles in CRPC therapy
- PMID: 39734237
- PMCID: PMC11684087
- DOI: 10.1186/s12951-024-03070-7
Targeting AURKA with multifunctional nanoparticles in CRPC therapy
Abstract
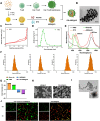
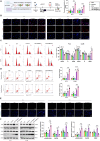
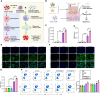
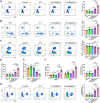
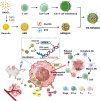
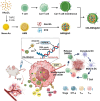
Castration-resistant prostate cancer (CRPC) presents significant therapeutic challenges due to its aggressive nature and poor prognosis. Targeting Aurora-A kinase (AURKA) has shown promise in cancer treatment. This study investigates the efficacy of ART-T cell membrane-encapsulated AMS@AD (CM-AMS@AD) nanoparticles (NPs) in a photothermal-chemotherapy-immunotherapy combination for CRPC. Bioinformatics analysis of the Cancer Genome Atlas-prostate adenocarcinoma (TCGA-PRAD) dataset revealed overexpression of AURKA in PCa, correlating with poor clinical outcomes. Single-cell RNA sequencing data from the GEO database showed a significant reduction in immune cells in CRPC. Experimentally, T cell membrane-biomimetic NPs loaded with the AURKA inhibitor Alisertib and chemotherapy drug DTX were synthesized and characterized by dynamic light scattering and transmission electron microscopy, showing good stability and uniformity (average diameter: 158 nm). In vitro studies demonstrated that these NPs inhibited CRPC cell proliferation, increased the G2/M cell population, and elevated apoptosis, confirmed by γH2AX expression. In vivo, CM-AMS@AD NPs accumulated in tumor tissues, significantly slowed tumor growth, decreased proliferation, increased apoptosis, and improved the immune environment, enhancing dendritic cell (DC) maturation and increasing CD8 + /CD4 + ratios. These findings suggest that CM-AMS@AD NPs offer a promising triple-combination therapy for CRPC, integrating photothermal, chemotherapy, and immunotherapy, with significant potential for future clinical applications.
Keywords: Aurora-A kinase (AURKA); Castration-resistant prostate cancer (CRPC); Chemotherapy; Immunotherapy; Nanoparticles; Photothermal therapy.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Study protocols were approved by the Ethics Committee of Ganzhou Hospital-Nanfang Hospital (Grant No.ZKY2023-107) and based on the ethical principles of the Declaration of Helsinki. The animal experiments were performed strictly per the guidelines in the Guide for the Care and Use of Laboratory Animals. The protocol of animal experiments was ratified by the Institutional Animal Care and Use Committee of Ganzhou Hospital-Nanfang Hospital (Grant No.DKY2023-019). Competing interests: The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
- 82360598/the National Natural Science Foundation of China
- WHWJ2023y002/the Wuhu Municipal Health Committee, Anhui, China
- 2024AH051878/the Higher Education Scientific Research Project of Anhui Province, China
- 202204295107020011/the Anhui Provincial Clinical Medicine Research Transformation Special Fund
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

