Angiotensin (1-7) Improves Pancreatic Islet Function via Upregulating PDX-1 and GCK: A Dose-Dependent Study in Mice
- PMID: 39734383
- PMCID: PMC11671625
- DOI: 10.1155/ije/1672096
Angiotensin (1-7) Improves Pancreatic Islet Function via Upregulating PDX-1 and GCK: A Dose-Dependent Study in Mice
Abstract
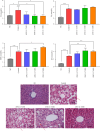
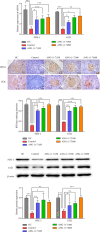
Purpose: This study aimed to verify the effect of angiotensin (1-7) on improving islet function and further explore the signaling pathway that may be involved in this improvement. It also aimed to explore the effects of angiotensin (1-7) on blood glucose levels, islet function, and morphological changes in db/db mice and its potential signal pathway. Methods: Forty-five db/db mice were divided randomly into a model control group and different doses of angiotensin (1-7) intervention groups (0, 150, 300, and 600 μg/kg/d), while seven db/m mice were assigned as the normal control group. The angiotensin (1-7) intervention groups received daily intraperitoneal administration for 8 weeks, whereas the normal control group was injected intraperitoneally with an equal volume of normal saline every day for 8 weeks. Changes in weight and food intake of mice were detected. Effect of angiotensin (1-7) on lipid metabolism, islet function, the morphology of pancreatic islets, and β-cell mass on mice were evaluated. The expression of PDX-1 and GCK in pancreatic tissue was verified. Results: The group receiving angiotensin (1-7) at a dosage of 600 μg/kg/d showed a significant decrease in body weight, triglyceride levels, and fasting blood glucose, along with an improvement in glucose tolerance. In the 300 μg/kg/d group, angiotensin (1-7) tended to increase the total volume of islets. Moreover, the intervention groups exhibited a significant increase in the ratio of β cells, small islets (30-80 μm in diameter), as well as the expression levels of PDX-1 and GCK in pancreatic tissue. Conclusion: Angiotensin (1-7) could improve glucose and lipid metabolism and islet function by promoting the expression of PDX-1 and GCK genes in the pancreas of db/db mice.
Keywords: GCK; PDX-1; angiotensin (1–7); diabetes mellitus; islet cells.
Copyright © 2024 Ziwei Lin et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

