Drug-induced retinal vein occlusion: a disproportionality analysis from the FDA adverse event reporting system (2004-2023)
- PMID: 39734405
- PMCID: PMC11671269
- DOI: 10.3389/fphar.2024.1480269
Drug-induced retinal vein occlusion: a disproportionality analysis from the FDA adverse event reporting system (2004-2023)
Abstract
Introduction: Retinal vein occlusion (RVO) often causes irreversible visual impairment, making early prevention crucial. This study aims to identify associations between different medications and RVO and provide information for clinical practice.
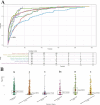
Method: This study included reports of RVO from the FDA Adverse Event Reporting System (FAERS) database from the first quarter (Q1) of 2004 to the fourth quarter (Q4) of 2023. The reported drugs were analyzed for adverse drug reaction (ADR) signals using four disproportionality algorithms. Kaplan-Meier curves and median time to onset were used to evaluate the drugs.
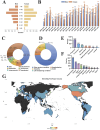
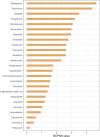
Results: From 2004 to 2023, the FAERS database recorded 6,151 reports associated with RVO. Disproportionality analyses identified 25 drugs significantly associated with RVO. Mirabegron showed the highest risk signal, followed by Raloxifene, Tadalafil, Fingolimod, and Bimatoprost. These high-risk drugs are distributed across different therapeutic areas, including urogenital system and sex hormones, ophthalmic drugs, nervous system drugs, musculoskeletal system drugs, anti-tumor and immune-modulating drugs, and anti-parasitic drugs. Specific drug targets such as adrenergic receptor agonists, hormone regulators, and PDE5 inhibitors were identified as high risk. Ophthalmic drugs exhibited the longest median time to adverse ocular reactions at 532.01 days, followed by anti-parasitic drugs, nervous system drugs, urogenital system and sex hormone drugs, anti-tumor and immune-modulating drugs, and musculoskeletal system drugs.
Conclusion: This study provides an overview of drug-induced RVO, identifying potential culprit drugs and their distribution characteristics. These findings enhance understanding of medication safety and help optimize clinical practice.
Keywords: FDA adverse event reporting system; adverse events; disproportionality analysis; pharmacovigilance; retinal vein occlusion.
Copyright © 2024 Chen, Xiao and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

