Preliminary study on the preparation of lyophilized acellular nerve scaffold complexes from rabbit sciatic nerves with human umbilical cord mesenchymal stem cells
- PMID: 39734476
- PMCID: PMC11669985
- DOI: 10.4252/wjsc.v16.i12.1047
Preliminary study on the preparation of lyophilized acellular nerve scaffold complexes from rabbit sciatic nerves with human umbilical cord mesenchymal stem cells
Abstract
Background: The gold standard of care for patients with severe peripheral nerve injury is autologous nerve grafting; however, autologous nerve grafts are usually limited for patients because of the limited number of autologous nerve sources and the loss of neurosensory sensation in the donor area, whereas allogeneic or xenografts are even more limited by immune rejection. Tissue-engineered peripheral nerve scaffolds, with the morphology and structure of natural nerves and complex biological signals, hold the most promise as ideal peripheral nerve "replacements".
Aim: To prepare allogenic peripheral nerve scaffolds using a low-toxicity decellularization method, and use human umbilical cord mesenchymal stem cells (hUC-MSCs) as seed cells to cultivate scaffold-cell complexes for the repair of injured peripheral nerves.
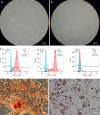
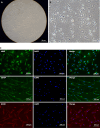
Methods: After obtaining sciatic nerves from New Zealand rabbits, an optimal acellular scaffold preparation scheme was established by mechanical separation, varying lyophilization cycles, and trypsin and DNase digestion at different times. The scaffolds were evaluated by hematoxylin and eosin (HE) and luxol fast blue (LFB) staining. The maximum load, durability, and elastic modulus of the acellular scaffolds were assessed using a universal material testing machine. The acellular scaffolds were implanted into the dorsal erector spinae muscle of SD rats and the scaffold degradation and systemic inflammatory reactions were observed at 3 days, 1 week, 3 weeks, and 6 weeks following surgery to determine the histocompatibility between xenografts. The effect of acellular scaffold extracts on fibroblast proliferation was assessed using an MTT assay to measure the cytotoxicity of the scaffold residual reagents. In addition, the umbilical cord from cesarean section fetuses was collected, and the Wharton's jelly (WJ) was separated into culture cells and confirm the osteogenic and adipogenic differentiation of mesenchymal stem cells (MSCs) and hUC-MSCs. The cultured cells were induced to differentiate into Schwann cells by the antioxidant-growth factor induction method, and the differentiated cells and the myelinogenic properties were identified.
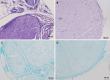
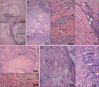
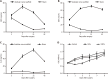
Results: The experiments effectively decellularized the sciatic nerve of the New Zealand rabbits. After comparing the completed acellular scaffolds among the groups, the optimal decellularization preparation steps were established as follows: Mechanical separation of the epineurium, two cycles of lyophilization-rewarming, trypsin digestion for 5 hours, and DNase digestion for 10 hours. After HE staining, no residual nuclear components were evident on the scaffold, whereas the extracellular matrix remained intact. LFB staining showed a significant decrease in myelin sheath composition of the scaffold compared with that before preparation. Biomechanical testing revealed that the maximum tensile strength, elastic modulus, and durability of the acellular scaffold were reduced compared with normal peripheral nerves. Based on the histocompatibility test, the immune response of the recipient SD rats to the scaffold New Zealand rabbits began to decline3 weeks following surgery, and there was no significant rejection after 6 weeks. The MTT assay revealed that the acellular reagent extract had no obvious effects on cell proliferation. The cells were successfully isolated, cultured, and passaged from human umbilical cord WJ by MSC medium, and their ability to differentiate into Schwann-like cells was demonstrated by morphological and immunohistochemical identification. The differentiated cells could also myelinate in vitro.
Conclusion: The acellular peripheral nerve scaffold with complete cell removal and intact matrix may be prepared by combining lyophilization and enzyme digestion. The resulting scaffold exhibited good histocompatibility and low cytotoxicity. In addition, hUC-MSCs have the potential to differentiate into Schwann-like cells with myelinogenic ability following in vitro induction.
Keywords: Acellular nerve scaffolds; Human umbilical cord mesenchymal stem cells; Peripheral nerve injury; Schwann cells.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no competing interests.
Figures



References
-
- Hale HB, Bae DS, Waters PM. Current concepts in the management of brachial plexus birth palsy. J Hand Surg Am. 2010;35:322–331. - PubMed
-
- Wang Q, Zhang C, Zhang L, Guo W, Feng G, Zhou S, Zhang Y, Tian T, Li Z, Huang F. The preparation and comparison of decellularized nerve scaffold of tissue engineering. J Biomed Mater Res A. 2014;102:4301–4308. - PubMed
-
- Sondell M, Lundborg G, Kanje M. Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res. 1998;795:44–54. - PubMed
-
- Hudson TW, Zawko S, Deister C, Lundy S, Hu CY, Lee K, Schmidt CE. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004;10:1641–1651. - PubMed
LinkOut - more resources
Full Text Sources

