Neurological manifestations of encephalitic alphaviruses, traumatic brain injuries, and organophosphorus nerve agent exposure
- PMID: 39734493
- PMCID: PMC11671522
- DOI: 10.3389/fnins.2024.1514940
Neurological manifestations of encephalitic alphaviruses, traumatic brain injuries, and organophosphorus nerve agent exposure
Abstract
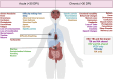
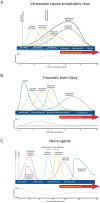
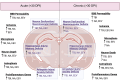
Encephalitic alphaviruses (EEVs), Traumatic Brain Injuries (TBI), and organophosphorus nerve agents (NAs) are three diverse biological, physical, and chemical injuries that can lead to long-term neurological deficits in humans. EEVs include Venezuelan, eastern, and western equine encephalitis viruses. This review describes the current understanding of neurological pathology during these three conditions, provides a comparative review of case studies vs. animal models, and summarizes current therapeutics. While epidemiological data on clinical and pathological manifestations of these conditions are known in humans, much of our current mechanistic understanding relies upon animal models. Here we review the animal models findings for EEVs, TBIs, and NAs and compare these with what is known from human case studies. Additionally, research on NAs and EEVs is limited due to their classification as high-risk pathogens (BSL-3) and/or select agents; therefore, we leverage commonalities with TBI to develop a further understanding of the mechanisms of neurological damage. Furthermore, we discuss overlapping neurological damage mechanisms between TBI, NAs, and EEVs that highlight novel medical countermeasure opportunities. We describe current treatment methods for reducing neurological damage induced by individual conditions and general neuroprotective treatment options. Finally, we discuss perspectives on the future of neuroprotective drug development against long-term neurological sequelae of EEVs, TBIs, and NAs.
Keywords: Venezuelan equine encephalitis virus; eastern equine encephalitis virus; neuroinflammation; neurological sequelae; organophosphorus nerve agent; traumatic brain injury; western equine encephalitis virus.
Copyright © 2024 VanderGiessen, de Jager, Leighton, Xie, Theus, Johnson and Kehn-Hall.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Machine learning identifies genes linked to neurological disorders induced by equine encephalitis viruses, traumatic brain injuries, and organophosphorus nerve agents.Front Comput Neurosci. 2025 May 13;19:1529902. doi: 10.3389/fncom.2025.1529902. eCollection 2025. Front Comput Neurosci. 2025. PMID: 40433315 Free PMC article.
-
Neurological Sequelae Resulting from Encephalitic Alphavirus Infection.Front Microbiol. 2016 Jun 20;7:959. doi: 10.3389/fmicb.2016.00959. eCollection 2016. Front Microbiol. 2016. PMID: 27379085 Free PMC article. Review.
-
Comparative pathology study of Venezuelan, eastern, and western equine encephalitis viruses in non-human primates.Antiviral Res. 2020 Oct;182:104875. doi: 10.1016/j.antiviral.2020.104875. Epub 2020 Aug 2. Antiviral Res. 2020. PMID: 32755661
-
A Monovalent and Trivalent MVA-Based Vaccine Completely Protects Mice Against Lethal Venezuelan, Western, and Eastern Equine Encephalitis Virus Aerosol Challenge.Front Immunol. 2021 Jan 19;11:598847. doi: 10.3389/fimmu.2020.598847. eCollection 2020. Front Immunol. 2021. PMID: 33542715 Free PMC article.
-
Understanding host responses to equine encephalitis virus infection: implications for therapeutic development.Expert Rev Anti Infect Ther. 2022 Dec;20(12):1551-1566. doi: 10.1080/14787210.2022.2141224. Epub 2022 Nov 4. Expert Rev Anti Infect Ther. 2022. PMID: 36305549 Review.
Cited by
-
Machine learning identifies genes linked to neurological disorders induced by equine encephalitis viruses, traumatic brain injuries, and organophosphorus nerve agents.Front Comput Neurosci. 2025 May 13;19:1529902. doi: 10.3389/fncom.2025.1529902. eCollection 2025. Front Comput Neurosci. 2025. PMID: 40433315 Free PMC article.
References
-
- AbuHasan Q., Reddy V., Siddiqui W. (2024). Neuroanatomy, Amygdala. Treasure Island, FL: StatPearls. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

