Autophagy mediated tubulobulbar complex components degradation is required for spermiation
- PMID: 39734555
- PMCID: PMC11670705
- DOI: 10.1016/j.fmre.2022.10.006
Autophagy mediated tubulobulbar complex components degradation is required for spermiation
Abstract
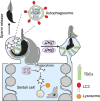
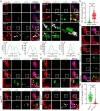
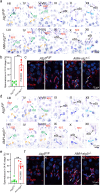
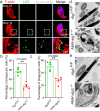
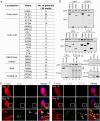
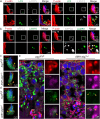
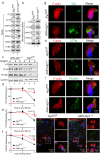
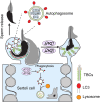
Spermiation is the process that releases mature spermatids from Sertoli cells into the lumen of the seminiferous tubule. Tubulobulbar complexes (TBCs) are elaborate cytoskeleton-related structures that are indispensable for spermiation. Despite well-defined ultrastructural events, the molecular regulation of TBCs during spermiation remains largely unknown. Here, we show that autophagy is active in TBC regions, and impaired autophagy in Sertoli cells affects spermiation. Further studies demonstrated that many TBC components bound to LC3 and could be selectively degraded through the autophagy-lysosome pathway. Perturbed autophagy impaired the degradation of some TBC components in Sertoli cells, such as VCL and CTTN, and led to the accumulation of TBC components surrounding the spermatid head, which may be associated with the sperm-releasing defect. Together, our results reveal that autophagy is essential for the TBC components degradation in mouse Sertoli cells and define a functional role of autophagy during spermiation.
Keywords: Autophagy; Cytoskeleton degradation; Lysosome; Sertoli cells; Spermiation; Tubulobulbar complexes.
© 2022 The Authors. Publishing Services by Elsevier B.V. on behalf of KeAi Communications Co. Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest in this work.
Figures
References
-
- Beardsley A., O'Donnell L. Characterization of normal spermiation and spermiation failure induced by hormone suppression in adult rats. Biol. Reprod. 2003;68:1299–1307. - PubMed
-
- Russell L.D. The perils of sperm release - let my children go. Int. J. Androl. 1991;14:307–311. - PubMed
-
- O'Donnell L., Narula A., Balourdos G., et al. Impairment of spermatogonial development and spermiation after testosterone-induced gonadotropin suppression in adult monkeys (Macaca fascicularis) J. Clin. Endocrinol. Metab. 2001;86:1814–1822. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

