Enhancing bone regeneration through 3D printed biphasic calcium phosphate scaffolds featuring graded pore sizes
- PMID: 39734570
- PMCID: PMC11681834
- DOI: 10.1016/j.bioactmat.2024.11.024
Enhancing bone regeneration through 3D printed biphasic calcium phosphate scaffolds featuring graded pore sizes
Abstract
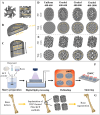
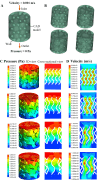
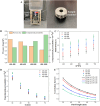
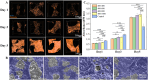
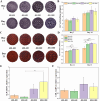
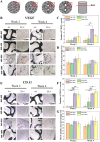
Human long bones exhibit pore size gradients with small pores in the exterior cortical bone and large pores in the interior cancellous bone. However, most current bone tissue engineering (BTE) scaffolds only have homogeneous porous structures that do not resemble the graded architectures of natural bones. Pore-size graded (PSG) scaffolds are attractive for BTE since they can provide biomimicking porous structures that may lead to enhanced bone tissue regeneration. In this study, uniform pore size scaffolds and PSG scaffolds were designed using the gyroid unit of triply periodic minimal surface (TPMS), with small pores (400 μm) in the periphery and large pores (400, 600, 800 or 1000 μm) in the center of BTE scaffolds (designated as 400-400, 400-600, 400-800, and 400-1000 scaffold, respectively). All scaffolds maintained the same porosity of 70 vol%. BTE scaffolds were subsequently fabricated through digital light processing (DLP) 3D printing with the use of biphasic calcium phosphate (BCP). The results showed that DLP 3D printing could produce PSG BCP scaffolds with high fidelity. The PSG BCP scaffolds possessed improved biocompatibility and mass transport properties as compared to uniform pore size BCP scaffolds. In particular, the 400-800 PSG scaffolds promoted osteogenesis in vitro and enhanced new bone formation and vascularization in vivo while they displayed favorable compressive properties and permeability. This study has revealed the importance of structural design and optimization of BTE scaffolds for achieving balanced mechanical, mass transport and biological performance for bone regeneration.
Keywords: 3D printing; Bone tissue engineering; Mass transport property; Mechanical property; Osteogenesis; Pore size graded scaffold; Vascularization.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Design and fabrication of biomimicking radially graded scaffolds via digital light processing 3D printing for bone regeneration.J Mater Chem B. 2023 Oct 25;11(41):9961-9974. doi: 10.1039/d3tb01573d. J Mater Chem B. 2023. PMID: 37818766
-
Influence of structural parameters of 3D-printed triply periodic minimal surface gyroid porous scaffolds on compression performance, cell response, and bone regeneration.J Biomed Mater Res B Appl Biomater. 2024 Jan;112(1):e35337. doi: 10.1002/jbm.b.35337. Epub 2023 Oct 5. J Biomed Mater Res B Appl Biomater. 2024. PMID: 37795764
-
Indirect selective laser sintering-printed microporous biphasic calcium phosphate scaffold promotes endogenous bone regeneration via activation of ERK1/2 signaling.Biofabrication. 2020 Mar 27;12(2):025032. doi: 10.1088/1758-5090/ab78ed. Biofabrication. 2020. PMID: 32084655
-
Additively manufactured porous scaffolds by design for treatment of bone defects.Front Bioeng Biotechnol. 2024 Jan 19;11:1252636. doi: 10.3389/fbioe.2023.1252636. eCollection 2023. Front Bioeng Biotechnol. 2024. PMID: 38312510 Free PMC article. Review.
-
Porosity of 3D biomaterial scaffolds and osteogenesis.Biomaterials. 2005 Sep;26(27):5474-91. doi: 10.1016/j.biomaterials.2005.02.002. Biomaterials. 2005. PMID: 15860204 Review.
Cited by
-
The bone marrow mesenchymal stem cells derived migrasomes induced by Titania nanotubes surface serve as chemotaxis effect for osteogenesis.J Nanobiotechnology. 2025 Aug 29;23(1):592. doi: 10.1186/s12951-025-03641-2. J Nanobiotechnology. 2025. PMID: 40877924 Free PMC article.
-
Strategic advances in Vat Photopolymerization for 3D printing of calcium phosphate-based bone scaffolds: A review.Bioact Mater. 2025 Jun 27;52:719-752. doi: 10.1016/j.bioactmat.2025.05.001. eCollection 2025 Oct. Bioact Mater. 2025. PMID: 40677755 Free PMC article. Review.
-
Application of 3D Printing Technology in Dentistry: A Review.Polymers (Basel). 2025 Mar 26;17(7):886. doi: 10.3390/polym17070886. Polymers (Basel). 2025. PMID: 40219277 Free PMC article. Review.
-
The application of tissue engineering strategies for uterine regeneration.Mater Today Bio. 2025 Feb 18;31:101594. doi: 10.1016/j.mtbio.2025.101594. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40070871 Free PMC article. Review.
-
Current State of Knowledge Regarding the Treatment of Cranial Bone Defects: An Overview.Materials (Basel). 2025 Apr 29;18(9):2021. doi: 10.3390/ma18092021. Materials (Basel). 2025. PMID: 40363522 Free PMC article. Review.
References
-
- Adithya S.P., Sidharthan D.S., Abhinandan R., Balagangadharan K., Selvamurugan N. Nanosheets-incorporated bio-composites containing natural and synthetic polymers/ceramics for bone tissue engineering. Int. J. Biol. Macromol. 2020;164:1960–1972. - PubMed
-
- Collins M.N., Ren G., Young K., Pina S., Reis R.L., Oliveira J.M. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv. Funct. Mater. 2021;31(21)
-
- Wu S., Liu X., Yeung K.W.K., Liu C., Yang X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R Rep. 2014;80:1–36.
LinkOut - more resources
Full Text Sources

