Novel transition metal-free synthetic protocols toward the construction of 2,3-dihydrobenzofurans: a recent update
- PMID: 39734577
- PMCID: PMC11672212
- DOI: 10.3389/fchem.2024.1470861
Novel transition metal-free synthetic protocols toward the construction of 2,3-dihydrobenzofurans: a recent update
Abstract
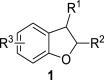
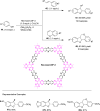
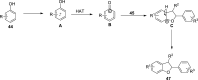
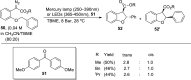
2,3-Dihydrobenzofurans are noteworthy scaffolds in organic and medicinal chemistry, constituting the structural framework of many of the varied medicinally active organic compounds. Moreover, a diverse variety of biologically potent natural products also contain this heterocyclic nucleus. Reflecting on the wide biological substantiality of dihydrobenzofurans, several innovative and facile synthetic developments are evolving to achieve these heterocycles. This review summarizes the transition-metal-free, efficient, and novel synthetic pathways toward constructing the dihydrobenzofuran nucleus established after 2020.
Keywords: catalyst free; dihydrobenzofurans; organocatalyzed; photocatalytic; transition metal-free.
Copyright © 2024 Mushtaq, Irfan, Haq, Mansha, Khan, Zahoor, Parveen, Irfan, Kotwica-Mojzych, Glowacka and Mojzych.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
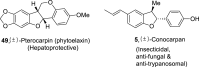
References
-
- Alexander H. (1892). Reduction des Cumarons. Ber. Dtsch. Chem. 25, 2409–2411. 10.1002/cber.18920250236 - DOI
Publication types
LinkOut - more resources
Full Text Sources

